Abstract
Neutron powder diffraction and thermoelectric characterization of SnSe:Kx intermetallic alloys are presented. Nanostructured ingots were prepared by arc-melting elemental tin and selenium along with potassium hydride. Up to x = 0.1 of K can be incorporated into SnSe. Rietveld refinement of the diffractograms locates potassium on the Sn site in the high-temperature Cmcm structure. However, in the low-temperature Pnma structure, K cannot be localized by difference Fourier maps, indicating the incorporation of K in a disordered form in the interlayer space. STEM-EELS indicates the incorporation of K into the SnSe grains. The resistivity upon K-doping at intermediate temperatures decreases by 1–2 orders of magnitude, but at high temperature is higher than the undoped SnSe. The Seebeck coefficient of K-doped SnSe remains p-type and almost temperature independent (400 μV/K for x = 0.1). The ultralow thermal conductivity of undoped SnSe decreases further upon K-doping to below 0.3 W/m K.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermoelectric materials hold the promise to help create a greener and eco-friendlier energy economy [1]. Thermoelectrics can convert temperature gradients into electrical voltage, or electric current to (negative) heat through the Seebeck or Peltier effect. Thermoelectrics are typically good semiconductors with large Seebeck coefficient (S), but they also need good electrical conductivity (σ), which requires a compromise by tuning the Fermi level with chemical doping. Furthermore, good thermoelectrics extract low thermal conductivity (κ), but since the Wiedemann–Franz law connects the electrical conductivity to the electronic thermal conductivity (κel), only the lattice thermal conductivity (κlatt) may be improved independently [2]. Nanostructuring is often the chosen strategy to decrease κlatt and preserve the electronic transport properties S and σ [3,4,5,6,7,8]. In the realm of material science, these competing properties are evaluated in terms of the dimensionless thermoelectric figure of merit zT = T * S2σ/(κel + κlatt).
Thermoelectrics offer versatile ways for applications, such as devices for waste heat to electricity conversion in thermoelectric generators, or Peltier coolers for reliable systems with no gases or moving parts [9, 10]. The full design from the synthesis of materials to the fabrication of devices must be assessed [11, 12]. For many applications such as powering sensors or for Peltier coolers, good room temperature thermoelectrics are needed [13].
There are several successful families of thermoelectrics that can provide both the required p- and n-type semiconductors for thermoelectric modules [14, 15]. Thermoelectrics are often made of environmentally toxic or rare elements. Finding cost-effective synthesis of environmentally friendlier nanostructured thermoelectrics has become a focus of recent research [16]. Nevertheless, some of the more successful types of thermoelectrics are based on tellurides [17], such as bismuth telluride (Bi2Te3) [4,5,6,7, 18,19,20,21,22], lead telluride (PbTe) [23,24,25] and germanium telluride (GeTe) [26,27,28,29,30,31]. Skutterudites are another attractive family of thermoelectrics, as they are high mobility semiconductors that chemically and structurally offer many ways of doping and property tuning [1, 32, 33]. They can be prepared by various methods in addition to conventional metallurgy, including high-pressure synthesis [34,35,36], melt spinning [37, 38], microwave synthesis [39], etc. As alternatives, oxides, such as SrTiO3 have been considered recently [40, 41].
There are multiple strategies to improve some of the thermoelectric properties in addition to nanostructuring, such as band engineering [42,43,44,45,46,47], energy filtering [48,49,50], the phonon glass electron crystal approach [51, 52] or high entropy design [53].
Tin selenide, SnSe, is a semiconductor with a quasi-2D laminar crystal structure resembling that of black phosphorous, also a potential thermoelectric [54]. In 2014, single crystals of SnSe were found to be the best thermoelectric up then with zT ~ 2.6 [55]. It has remarkable 3D charge but 2D phonon transport properties due to its crystal structure [56], anharmonicity, multiple valence band along with a continuous phase transition near maximum zT [57]. Polycrystalline SnSe can be prepared by several synthesis techniques [58], among them with co-doping and multi-nanoprecipitates [59], with aqueous synthesis [60], as well as arc-melting. SnSe films can also show good thermoelectric performance [61,62,63]. Successful thermoelectric generators have been made based on SnSe very recently [64]. The effects of vacancy on the thermoelectric properties have been studied in detail [65,66,67,68]. Applied pressure transforms SnSe into a semimetal [69]. SnSe can be doped with a wide variety of elements, including Ge [70,71,72], Sb [73], or alloyed with SnTe [8] and SnS [74].
Arc-melting produces highly nanostructured SnSe efficiently [3] and is a versatile synthesis procedure for various dopings [70, 75]. This synthesis produces material with elemental ratios or doping different from the nominal amount, as either dopant, tin or selenide is easily lost to the arc [73, 76]. The polycrystalline n-type SnSe pellets produced by arc-melting can reach high zT up to 1.8 [77].
High-performance p-type SnSe polycrystal may be stabilized by alkali doping (with Li, Na and K) [78,79,80], as well as Na-doped SnSe1−xTex [81]. In alkali-doped SnSe, the location of the dopant is crucial. As stated by Wei et al., “…actual occupancy (i.e., substitutional, interstitial, or intercalated type) of these dopants is an important concern" [78]. Alkali doping may be beneficial in removing or preventing the formation of the high-thermal conductivity intergrain tin oxide layers and forming coherent nanoprecipitates [82]. In conventional synthesis techniques, the handling and introduction of highly flammable and air-sensitive alkali metals poses a technical nuisance.
Here we report the synthesis of SnSe:Kx intermetallic alloys, using potassium hydride, and their high resolution neutron powder diffraction (NPD) investigation directed to characterize the position of the K dopant, along with the thermoelectric properties. The advantage of using KH as starting material to introduce K has to do with its easy handling, avoiding issues with ambient oxidation, with the expectation that H2 is released and lost during synthesis. A similar method, using KH, was employed recently to produce K-filled CoSb3 skutterudite under high pressure [32]. The synthesis may also help avoiding the formation of tin oxide, which has been shown to be responsible for an undesired increase of effective thermal conductivity [83,84,85].
Materials and methods
SnSe:Kx intermetallic alloys were prepared in an Edmund Buhler MAM-1 mini-arc furnace. A mixture of stoichiometric amounts of Sn and Se powder metals, and KH as a source of K, was pelletized under N2 atmosphere in a glove box; the pellets were arc-molten under Ar atmosphere in a water-cooled Cu crucible, leading to compact ingots, which were ground to powder for structural characterization, or cold-pressed into regular disk-shaped specimens for transport measurements. Cold-pressing SnSe is sufficient for decent thermoelectric performance [77, 86]; it provides samples with about 90% of the crystallographic density thanks to its malleability. On the contrary, more typical consolidation techniques, such as hot pressing [87] or spark plasma sintering [21, 85], would yield higher densities but tend to deteriorate the electrical and thermal conductivities, mainly by causing intergrain oxide growth [88].
Neutron powder diffraction (NPD) data were taken in the D2B diffractometer at the Institut Laue-Langevin, Grenoble, in the high-resolution configuration, with a neutron wavelength of λ = 1.594 Å. About 2 g of the sample were contained in a vanadium can and placed in the isothermal zone of a furnace with a vanadium resistor operating under vacuum (PO2 ≈ 10–6 Torr residual pressure). The measurements were carried out upon warming at 295, 523, 673, 773, 873 and 973 K. The NPD patterns were collected for 2 h in the high-flux mode. The diffraction patterns were analyzed by the Rietveld method with the FULLPROF program [89]. The line shape of the diffraction peaks was generated by a pseudo-Voigt function. The following parameters were refined: background points, zero shift, half width, pseudo-Voigt, scale factor and unit-cell parameters. Positional and occupancy factors and isotropic displacement factors were also refined. The coherent scattering lengths for Sn, K and Se were, respectively, 6.225, 3.670 and 7.970 fm. A preferred orientation correction was applied, considering platelets perpendicular to the [100] direction.
All the thermoelectric properties were measured along the pressing direction, where previous reports show a higher ZT [90,91,92]. Seebeck coefficient and four-probe resistivity measurements were carried out in high vacuum (10–6 mbar) in a commercial MMR instrument. Thermal conductivity was determined from the thermal diffusivity (α) using a Linseis LFA 1000 instrument by a laser-flash technique, as \(\kappa = \alpha ,C_{{\text{p}}} ,d\), where Cp = 252–263 J/kg K is the specific heat calculated using the Dulong–Petit equation, and d = 5.5–6.0 g/cm3 is the sample density (90–96% of the crystallographic value), for the various compositions between x = 0–0.1.
Scanning transmission electron microscopy (STEM) is carried out in a JEOL ARM 200 electron microscope operated at 200 kV. The microscope is equipped with a Gatan Quantum electron energy loss spectrometer (EELS). Transmission electron microscopy (TEM) image S7 is done in JEOL 3000F electron microscope operated at 300 kV.
Results
X-ray diffraction
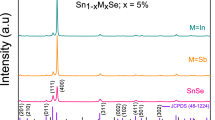
SnSe:Kx specimens were obtained by arc-melting as robust ingots, ground into black, well-crystallized powders in order to perform the structural characterization. Typical diffraction patterns are affected by a certain preferred orientation [3, 93]. Figure 1 shows the XRD patterns of SnSe:Kx samples (x = 0, 0.0.25, 0.05, 0.0.75, 0.1) including that of the parent SnSe compound. All the diagrams correspond to well-crystallized GeSe-like structures with orthorhombic geometry defined in the space group Pnma.
The crystal structures were initially refined from XRD data in order to follow the evolution of the lattice parameters upon K doping. Figure 2 displays the mentioned variation with x (nominal amount of K). The unit-cell sizes substantially differ from that of the parent SnSe compound (with unit-cell parameters: a = 11.5067(1), b = 4.1551(1) and c = 4.4475(2) Å), showing an overall increment of a, b and c parameters and unit-cell volume, V, as K is introduced into the crystal structure up to a maximum of x = 0.1. This expansion of the lattice happens because the atomic size of K+ is bigger than that of Sn2+. Beyond this doping level (nominal x = 0.15, 0.2), the size of the cell decreases again. Figure S6 (in the Supplementary Information) displays the XRD pattern of the x = 0.2 material, showing the appearance of some impurities (unidentified) in the low Bragg-angle region.
Therefore, we consider that x = 0.1 is the maximum K content the system is able to accept under the current preparation conditions and, thus, the x = 0.15 and 0.2 specimens were excluded from this study.
In-situ NPD study
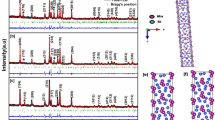
The investigation of the crystal structure evolution by neutron powder diffraction (NPD) of the sample with the maximum K doping level (x = 0.1) was essential since the bulk analysis provided by neutrons is exceptionally good to minimize the preferred orientation effect, given the packing of the ground crystals in vanadium cylinders. Furthermore, neutrons allow us to explore a much wider range of the reciprocal space, whereas the lack of form factor permits the precise determination of the site occupancy and anisotropic displacement factors, since the neutron scattering lengths do not decrease with the scattering angle. Figure 3a, b show the NPD patterns collected at 295 K and 873 K, respectively; a symmetry increase is observed, since a structural phase transition from Pnma to Cmcm space groups occurs between the 673 K and the 773 K patterns (represented in the SI). In fact, Pnma is a subgroup of Cmcm, retaining half of the space group symmetry elements. For the room-temperature pattern (T = 295 K), the crystal structure was Rietveld-refined in the Pnma space group (No. 62) with a = 11.5132 (8) Å, b = 4.1538 (3) Å, c = 4.4438 (3) Å and V = 212.52 (2) Å3. Both Sn and Se atoms are located at 4c (x, ¼, z) positions. Figure 4 shows a sketch with the crystal structures defined in the Pnma (low-temperature) and Cmcm (high-temperature).
Crystal structure of SnSe:K0.1 for the low-temperature phase defined in Pnma (left panel) and high-temperature phase (right panel). In the former, K is thought to reside in the interlayer space, whereas in the latter K is incorporated to the Sn positions. The coordination polyhedra in Pnma is defined as trigonal pyramids SnSe3, whereas in Cmcm they are (Sn,K)Se5 polyhedra
Despite the net expansion observed with respect to pristine SnSe at RT, the localization of K atoms was not possible from NPD data: trial refinements with K at the Sn sites led to slightly negative occupancy factors for K. Difference Fourier maps did not show a clear location of the additional atoms, and therefore we assume that the expansion affecting mainly the a parameter (perpendicular to the corrugated layers of SnSe) is due to the incorporation of K in a disordered form in the interlayer space. This configuration is suggested in Fig. 4. Table S1 (Supporting information) includes the atomic parameters and displacements factors at 295 K.
The refinement of the 573 K and 673 K structures was also carried out in the Pnma space group; the patterns did not show symptoms of deviations from that symmetry and space group. The results of those refinements are included in Tables S2 and S3; the quality of the Rietveld plots is shown in Figs. S1 and S2, also corresponding to excellent agreement factors.
The left panel of Fig. 4 depicts the orthorhombic Pnma low-temperature phase, consisting of puckered layers of corner sharing SnSe3 polyhedra, perpendicular to the a unit-cell parameter. Selenium atoms also form SeSn3 trigonal pyramids. As indicated above, the K atoms could not be identified at Sn sites, so it is inferred that they occupy the interlayer space, driving the observed expansion of the a unit-cell parameter.
The 773 K, the NPD pattern already shows a mixture of the low-temperature Pnma and high-temperature Cmcm phases, described below (Fig. S3). The full refinement was performed with both structural models; from the scale factors we could determine 66% of Pnma phase and 33% of Cmcm coexisting in equilibrium during the two hours of data acquisition at 773 K. The 873 K pattern (Fig. S4) already corresponds to a pure Cmcm phase, as well as the 973 K diagram (Fig. S5); the crystal structure was modeled as belonging to the TlI family [94] with both Sn and Se atoms at 4c (0, ¼, z) positions. Table S4 includes the results of the simultaneous refinement of both Pnma and Cmcm phases identified at 773 K, and Tables S5 and S6 contain the structural details for the high-temperature phase at 873 K and 973 K, with unit-cell parameters a = 4.3132 (12) Å, b = 11.750 (3) Å, c = 4.3297 (13) Å, V = 219.42 (11) Å3.
Figure 4 (right panel) displays a view of the Cmcm crystal structure at 873 K. It is also a layered structure showing a more regular arrangement of Sn and Se atoms, this time exhibiting SnSe5 polyhedra conformed within each layer (perpendicular to the b axis). Very interestingly, in the case of the high-temperature phase defined in the Cmcm space group, the mixed refinement of the Sn,K positions led to positive occupancy factors for K, suggesting that this element stored in the interlayer space in the low-temperature phase is assimilated into the Sn positions in the high-temperature phase, probably because of the superior coordination number of this crystal structure. Both phases coexist at the same temperature of 773 K, indicating the sudden formation of strongly intra-layer covalent bonds between Sn and Se, therefore weakening the Van-der-Waals bonds between layers.
Figure 5 depicts the phase diagram displaying the thermal evolution of the unit-cell parameters of both orthorhombic phases. Upon heating, the b and c unit cell parameters of the Pnma space group tend to become identical, giving rise to similar values of c and a parameters in the Cmcm setting. The a parameter in Pnma, perpendicular to the puckered layers of SnSe3 pyramids, regularly increases upon heating, even across the phase transition, yielding a correspondingly higher b parameter in Cmcm.
Scanning transmission electron microscopy (STEM) confirms that potassium intercalates into the SnSe crystal in the form of random defects. The K-SnSe polycrystalline grains are observed to be a few micrometers in size. We have investigated more than a dozen grains by the means of electron energy loss spectroscopy (EELS). Figure 6 shows a chemical composition of a nanometric part of one K-SnSe grain. Panel (a) shows the region of the grain where EELS spectra are taken. The EELS spectra shown here cover just the energy range for potassium and tin edge: selenium L2,3 edge at 1436 eV has been detected in different EELS spectra (not shown). The grains are of high purity and contain only potassium (K—L2,3 edge) and tin (Sn—M4,5 edge), as shown in a typical EELS spectrum—panel b. Panels (c) and (d) show the relative composition of K and Sn. Note that the maximum K/Sn ratio of 15/85 in this two-elements quantification yields the potassium concentration of 8.1% when all three elements are taken into the account. In conclusion, potassium is distributed inhomogeneously in the crystal matrix of SnSe with the concentrations between 0 and 9%.
The K–SnSe grain grains are highly polycrystalline and difficult to orient for the atomic resolution imaging. Figure S7 shows the high-resolution transmission electron microscopy (HR-TEM) image of a small part (20 × 22 nm) of the K–SnSe grain. The lattice parameters deduced from this image are in agreement with the values found from the neutron powder diffraction. From the contrast patterns in Fig. S7, one can see that the left part of the image is oriented nearly on the zone axis, while the right part is of different crystal orientation. Additionally, the wavelike patterns on the upper left side are the same as the “Moiré pattern” in the SnSe undulated crystal, as reported in Gainza et al. [73]. In general, Fig. S7 indicates that the title material has the same Pnma crystal structure, and the nanostructuring is similar as in as other weakly doped SnSe crystals.
Thermoelectric transport properties
The thermoelectric transport properties of the SnSe:Kx series are plotted in Fig. 7. The resistivity near room temperature is about one order of magnitude lower than the resistivity of pristine SnSe; however, at high temperature, the undoped compound shows a dramatic decrease that is not mirrored by the samples doped with potassium. Among them, SnSe:K0.05 shows the lowest resistivity, reaching 10–3 Ω m at 780 K, whereas the Seebeck coefficient of the undoped SnSe made by arc-melting shows a change of sign reflecting a p-ton-type transition [77], it does not happen in any of the K-doped compounds, as they remain firmly p-type. The Seebeck coefficient of the SnSe:Kx samples remains at around ~ 200 µV/K until it drops above ~ 700 K, indicating the start of bipolar conduction. However, the SnSe:K0.1 compound has a higher Seebeck coefficient growing above 400 µV/K, with no sign of bipolar transport. Even this is low though compared to the K-doped SnSe prepared by ball-milling, which shows a Seebeck coefficient of 500–600 µV/K for the 8–12-h milled samples [82]. However, both resistivity and Seebeck coefficient behave very similar to that reported for Sn0.99K0.01Se prepared by melting in quartz tubes [78]. The maximum power factor, S2σ = 0.07 mW/m K2, is obtained in the SnSe:K0.05 composition at 700 K, comparable to that measured in the undoped sample at the same temperature.
Temperature dependance of the main thermoelectric properties of SnSe:Kx polycrystals prepared by arc-melting, a electrical resistivity, b seebeck eoefficient and c power factor. We have added the data of the undoped arc-melted SnSe for the sake of comparison, as extracted from Gainza et al. [77]
The thermal conductivity of each SnSe:Kx sample is very similar, as shown in Fig. 8. There are no significant differences between the total thermal conductivity of all the prepared compositions. In every case, the thermal conductivity of the K-doped samples is lower than that of undoped SnSe, but they all follow the same behavior corresponding to Umklapp process dominated phonon scattering [95], ~ αT−1. The lattice thermal conductivity is almost entirely the total thermal conductivity, since the electronic thermal conductivity is irrelevant below ~ 800 K in these arc-melted tin selenides [77]. For all the samples, we obtain an ultra-low thermal conductivity [57] (< 0.4–0.5 W/m K) at high temperature, an important feature for high-performance thermoelectric materials, probably due to the absence of tin oxides at the grain boundaries, promoted by the potassium doping [82], as well as the strong and multifaceted nanostructuring (see Fig. S7), already seen in the undoped compound [73].
The figure of merit of SnSe:Kx compounds is displayed in Fig. 9. The ZT peak is reached at ~ 700 K, with a maximum value of ~ 0.15 for the Sn0.95K0.05Se compound. This ZT shown by our non-optimized samples is similar to other values reported for SnSe doped with different alkali elements, with figures of merit around 0.2–0.4 at 700 K [78].
Figure of merit of the arc-melted SnSe:Kx. The peak is reached at 726 K, although is far from the peak of the undoped SnSe (~ 1.8 at 816 K, not shown [77])
A single parabolic band (SPB) analysis of the measured results is summarized in Figs. 10 and 11. Figure 10a shows the Figure of Merit as a function of the reduced chemical potential, η. This relation, determined by the thermoelectric quality factor B [96, 97], is a very useful tool to assess the potential performance of a thermoelectric material regardless of doping. The chemical potential is a magnitude that we can modify, in some measure, with proper doping. According to the analysis, for SnSe:K0.1 the Fermi-level lies far from the optimum position. This simple model predicts a ZT of 0.2 for the SnSe:K0.1 and SnSe:K0.05 compounds at 723 K with properly modified chemical potential. This maximum value is in line with some data reported for SnSe doped with 1% K, which shows a figure of merit of ~ 0.4 at 700 K [78, 82].
The weighted mobility is displayed in Fig. 11a. Although it seems to vary a lot with temperature, the weighted mobility is below 5 cm2/V s in all the compositions up to 800 K. This value is quite lower than that reported or PbTe or Mg3Sb1.5Bi1.5 (above 100 cm2/V s) [98]. The variations observed for the SnSe:K0.1 come from the slight variations of the resistivity with temperature, that can be observed in Fig. 7a. However, the net weighted mobility is very low at all temperatures, so these variations do not affect the transport analysis.
The electronic quality factor [99] for the compositions with potassium reaches a higher value than that of the undoped compound, as shown in Fig. 11b. The electronic quality factor for the SnSe:K0.025 remains almost the same, making clear that a certain minimum quantity of potassium doping is needed in order to observe significant changes in the carrier transport.
Conclusions
Summarizing, we have demonstrated that K doping has important repercussions on the transport properties of SnSe, which may be articulated as a function of some of the structural properties unveiled from neutron diffraction data. We observed that below the Pnma-to-Cmcm structural phase transition, K ions cannot be located by diffraction methods, but the conspicuous expansion along the a axis suggests that they are present in the interlayer space, also confirmed by STEM-EELS spectroscopic images. The transition to the more symmetric Cmcm structure, where the coordination index increases from 3 to 5, facilitates the incorporation of large K+ ions into the Sn positions, where they can indeed be located from NPD data. This incorporation drives important consequences concerning the electronic transport, accounting for the comparatively much higher resistivity above 700 K, where the K migration from the interlayer space to the Sn positions is triggered by the phase transition. Indeed, the electronic resistivity of pristine SnSe reaches values inferior by two orders of magnitude to the doped specimen at 800 K, which explains the poor Figure of Merit reached by K-doped specimens. At the same time, the characteristic change of sign of the Seebeck coefficient is also altered by the partial occupancy of Sn sites by K atoms. Finally, the K doping results in an additional decrease of the thermal conductivity, probably as a consequence of the structural disordering induced by the substitutional incorporation of K.
Supplemental information
Supplemental Information includes Supplemental Experimental Data on crystal structure, and calculations based on the single parabolic band model (SPB) of the predicted maximum ZT figure of merit, weighted mobility and electronic quality factor in 3 Figures and 1 Table can be found with this article online at [link inserted by JMS].
Data and code availability
The authors declare that data supporting the findings of this work are available within the article and the Supplemental Information. Any other data are available from the Lead Contact upon request.
Materials availability
This study did not generate new unique reagents. K-doped SnSe may be available upon request.
References
Beretta D, Neophytou N, Hodges JM et al (2019) Thermoelectrics: from history, a window to the future. Mater Sci Eng R Rep 138:210–255. https://doi.org/10.1016/j.mser.2018.09.001
Snyder GJ, Toberer ES (2008) Complex thermoelectric materials. Nat Mater 7:105–114. https://doi.org/10.1038/nmat2090
Serrano-Sánchez F, Gharsallah M, Nemes NM et al (2015) Record seebeck coefficient and extremely low thermal conductivity in nanostructured SnSe. Appl Phys Lett 106:083902. https://doi.org/10.1063/1.4913260
Serrano-Sánchez F, Gharsallah M, Nemes NM et al (2017) Enhanced figure of merit in nanostructured (Bi, Sb)2Te3 with optimized composition, prepared by a straightforward arc-melting procedure. Sci Rep 7:6277. https://doi.org/10.1038/s41598-017-05428-4
Akshay VR, Suneesh MV, Vasundhara M (2017) Tailoring thermoelectric properties through structure and morphology in chemically synthesized n-type bismuth telluride nanostructures. Inorg Chem 56:6264–6274. https://doi.org/10.1021/acs.inorgchem.7b00336
Akshay VR, Arun B, Suneesh MV, Vasundhara M (2018) Surfactant-induced structural phase transitions and enhanced room temperature thermoelectric performance in n-type Bi 2 Te 3 nanostructures synthesized via chemical route. ACS Appl Nano Mater 1:3236–3250. https://doi.org/10.1021/acsanm.8b00464
Mamur H, Bhuiyan MRA, Korkmaz F, Nil M (2018) A review on bismuth telluride (Bi2Te3) nanostructure for thermoelectric applications. Renew Sustain Energy Rev 82:4159–4169. https://doi.org/10.1016/j.rser.2017.10.112
Sidharth D, Alagar Nedunchezhian AS, Rajkumar R et al (2019) Enhancing effects of Te substitution on the thermoelectric power factor of nanostructured SnSe 1–x Te x. Phys Chem Chem Phys 21:15725–15733. https://doi.org/10.1039/C9CP02018G
He R, Schierning G, Nielsch K (2018) Thermoelectric devices: a review of devices, architectures, and contact optimization. Adv Mater Technol 3:1700256. https://doi.org/10.1002/admt.201700256
Champier D (2017) Thermoelectric generators: a review of applications. Energy Convers Manag 140:167–181. https://doi.org/10.1016/j.enconman.2017.02.070
Shi X-L, Zou J, Chen Z-G (2020) Advanced thermoelectric design: from materials and structures to devices. Chem Rev 120:7399–7515. https://doi.org/10.1021/acs.chemrev.0c00026
Wolf M, Rybakov A, Hinterding R, Feldhoff A (2020) Geometry optimization of thermoelectric modules: deviation of optimum power output and conversion efficiency. Entropy 22:1233. https://doi.org/10.3390/e22111233
Soleimani Z, Zoras S, Ceranic B et al (2021) The cradle to gate life-cycle assessment of thermoelectric materials: a comparison of inorganic, organic and hybrid types. Sustain Energy Technol Assessments 44:101073. https://doi.org/10.1016/j.seta.2021.101073
Nolas G, Woods LM, Funahashi R (2020) Advanced thermoelectrics. J Appl Phys 127:060401. https://doi.org/10.1063/1.5144998
Hasan MN, Wahid H, Nayan N, Mohamed Ali MS (2020) Inorganic thermoelectric materials: a review. Int J Energy Res 44:6170–6222. https://doi.org/10.1002/er.5313
Jaldurgam FF, Ahmad Z, Touati F (2021) Synthesis and performance of large-scale cost-effective environment-friendly nanostructured thermoelectric materials. Nanomaterials 11:1091. https://doi.org/10.3390/nano11051091
Lin S, Li W, Chen Z et al (2016) Tellurium as a high-performance elemental thermoelectric. Nat Commun 7:10287. https://doi.org/10.1038/ncomms10287
Gharsallah M, Serrano-Sánchez F, Bermúdez J et al (2016) Nanostructured Bi2Te3 prepared by a straightforward arc-melting method. Nanoscale Res Lett 11:4–10. https://doi.org/10.1186/s11671-016-1345-5
Witting IT, Chasapis TC, Ricci F et al (2019) The thermoelectric properties of bismuth telluride. Adv Electron Mater 5:1800904. https://doi.org/10.1002/aelm.201800904
Witting IT, Ricci F, Chasapis TC et al (2020) The thermoelectric properties of n-type bismuth telluride: bismuth selenide alloys Bi2Te(3–x)Se(x). Research 2020:1–15. https://doi.org/10.34133/2020/4361703
Han L, Spangsdorf SH, Nong NV et al (2016) Effects of spark plasma sintering conditions on the anisotropic thermoelectric properties of bismuth antimony telluride. RSC Adv 6:59565–59573. https://doi.org/10.1039/C6RA06688G
Bano S, Govind B, Kumar A, Misra DK (2020) Ni-doped Bi0.5Sb1.5Te3 single crystal: a potential functional material for thermoelectricity, topological insulator, and optoelectronics. J Mater Sci Mater Electron 31:15652–15658. https://doi.org/10.1007/s10854-020-04128-6
Gainza J, Serrano-Sánchez F, Biskup N et al (2019) Influence of nanostructuration on PbTe alloys synthesized by arc-melting. Materials (Basel) 12:3783. https://doi.org/10.3390/ma12223783
Brod MK, Snyder GJ (2021) Orbital chemistry of high valence band convergence and low-dimensional topology in PbTe. J Mater Chem A 9:12119–12139. https://doi.org/10.1039/D1TA01273H
Zhao LD, Wu HJ, Hao SQ et al (2013) All-scale hierarchical thermoelectrics: MgTe in PbTe facilitates valence band convergence and suppresses bipolar thermal transport for high performance. Energy Environ Sci 6:3346–3355. https://doi.org/10.1039/c3ee42187b
Serrano-Sánchez F, Funes M, Nemes NM et al (2018) Low lattice thermal conductivity in arc-melted GeTe with Ge-deficient crystal structure. Appl Phys Lett 113:1–5. https://doi.org/10.1063/1.5043359
Gainza J, Serrano-Sánchez F, Nemes NM et al (2019) Features of the high-temperature structural evolution of GeTe thermoelectric probed by neutron and synchrotron powder diffraction. Metals (Basel) 10:48. https://doi.org/10.3390/met10010048
Gao W, Liu Z, Zhang W et al (2021) Improved thermoelectric performance of GeTe via efficient yttrium doping. Appl Phys Lett 118:033901. https://doi.org/10.1063/5.0038957
Liu Z, Gao W, Zhang W et al (2020) High power factor and enhanced thermoelectric performance in Sc and Bi codoped GeTe: insights into the hidden role of rhombohedral distortion degree. Adv Energy Mater 10:2002588. https://doi.org/10.1002/aenm.202002588
Shuai J, Tan XJ, Guo Q et al (2019) Enhanced thermoelectric performance through crystal field engineering in transition metal-doped GeTe. Mater Today Phys 9:100094. https://doi.org/10.1016/j.mtphys.2019.100094
Dangić Đ, Hellman O, Fahy S, Savić I (2021) The origin of the lattice thermal conductivity enhancement at the ferroelectric phase transition in GeTe. NPJ Comput Mater 7:57. https://doi.org/10.1038/s41524-021-00523-7
Gainza J, Serrano-Sánchez F, Rodrigues JE et al (2020) Unveiling the correlation between the crystalline structure of M-filled CoSb 3 (M = Y, K, Sr) skutterudites and their thermoelectric transport properties. Adv Funct Mater 30:2001651. https://doi.org/10.1002/adfm.202001651
Liu Z-Y, Zhu J-L, Tong X et al (2020) A review of CoSb3-based skutterudite thermoelectric materials. J Adv Ceram. https://doi.org/10.1007/s40145-020-0407-4
Gainza J, Serrano-Sánchez F, Prado-Gonjal J et al (2019) Substantial thermal conductivity reduction in mischmetal skutterudites Mm x Co 4 Sb 12 prepared under high-pressure conditions, due to uneven distribution of the rare-earth elements. J Mater Chem C 7:4124–4131. https://doi.org/10.1039/C8TC06461J
Serrano-Sánchez F, Prado-Gonjal J, Nemes NM et al (2017) Low thermal conductivity in La-filled cobalt antimonide skutterudites with an inhomogeneous filling factor prepared under high-pressure conditions. J Mater Chem A 6:118–126. https://doi.org/10.1039/c7ta08545a
Gainza J, Serrano-Sánchez F, Rodrigues JEFS et al (2021) Metastable materials accessed under moderate pressure conditions (P ≤ 3.5 GPa) in a piston-cylinder press. Materials (Basel) 14:1946. https://doi.org/10.3390/ma14081946
Geng H, Zhang J, He T et al (2020) Microstructure evolution and mechanical properties of melt spun skutterudite-based thermoelectric materials. Materials (Basel) 13:984. https://doi.org/10.3390/ma13040984
Lee KH, Bae SH, Choi S-M (2019) Phase formation behavior and thermoelectric transport properties of P-Type YbxFe3CoSb12 prepared by melt spinning and spark plasma sintering. Materials (Basel) 13:87. https://doi.org/10.3390/ma13010087
Lei Y, Gao W, Li Y et al (2018) Structure and thermoelectric performance of Ti-filled and Te-doped skutterudite TixCo4Sb11.5Te0.5 bulks fabricated by combination of microwave synthesis and spark plasma sintering. Mater Lett 233:166–169. https://doi.org/10.1016/j.matlet.2018.08.157
Shi X-L, Wu H, Liu Q et al (2020) SrTiO3-based thermoelectrics: progress and challenges. Nano Energy 78:105195. https://doi.org/10.1016/j.nanoen.2020.105195
Prado-Gonjal J, López C, Pinacca R et al (2020) Correlation between crystal structure and thermoelectric properties of Sr1−xTi0.9Nb0.1O3−δ ceramics. Curr Comput-Aided Drug Des 10:100. https://doi.org/10.3390/cryst10020100
Heremans JP, Jovovic V, Toberer ES et al (2008) Enhancement of thermoelectric efficiency in pbte by distortion of the electronic density of states. Science 321:554–557. https://doi.org/10.1126/science.1159725
Zheng Z-H, Shi X-L, Ao D-W et al (2021) Rational band engineering and structural manipulations inducing high thermoelectric performance in n-type CoSb3 thin films. Nano Energy 81:105683. https://doi.org/10.1016/j.nanoen.2020.105683
Xie G, Li Z, Luo T et al (2020) Band inversion induced multiple electronic valleys for high thermoelectric performance of SnTe with strong lattice softening. Nano Energy 69:104395. https://doi.org/10.1016/j.nanoen.2019.104395
Pei Y, Tan G, Feng D et al (2017) Integrating band structure engineering with all-scale hierarchical structuring for high thermoelectric performance in PbTe system. Adv Energy Mater 7:1601450. https://doi.org/10.1002/aenm.201601450
Pei Y, Wang H, Snyder GJ (2012) Band engineering of thermoelectric materials. Adv Mater 24:6125–6135. https://doi.org/10.1002/adma.201202919
Imasato K, Kang SD, Ohno S, Snyder GJ (2018) Band engineering in Mg3Sb2 by alloying with Mg3Bi2 for enhanced thermoelectric performance. Mater Horizons 5:59s–564. https://doi.org/10.1039/c7mh00865a
Gayner C, Amouyal Y (2019) Energy filtering of charge carriers: current trends, challenges, and prospects for thermoelectric materials. Adv Funct Mater 1901789:1901789. https://doi.org/10.1002/adfm.201901789
Narducci D, Selezneva E, Cerofolini G et al (2012) Impact of energy filtering and carrier localization on the thermoelectric properties of granular semiconductors. J Solid State Chem 193:19–25. https://doi.org/10.1016/j.jssc.2012.03.032
Zide JMO, Vashaee D, Bian ZX et al (2006) Demonstration of electron filtering to increase the Seebeck coefficient in In0.53 Ga0.47 As/In0.53 Ga0.28 Al0.19 As superlattices. Phys Rev B 74:205335. https://doi.org/10.1103/PhysRevB.74.205335
Nolas GS, Sharp J, Goldsmid HJ (2001) The Phonon—Glass Electron-Crystal Approach to Thermoelectric Materials Research. Springer, Berlin, pp 177–207
Zhang J, Xu B, Wang L-M et al (2012) High-pressure synthesis of phonon-glass electron-crystal featured thermoelectric LixCo4Sb12. Acta Mater 60:1246–1251. https://doi.org/10.1016/j.actamat.2011.10.059
Cai J, Yang J, Liu G et al (2021) Ultralow thermal conductivity and improved ZT of CuInTe2 by high-entropy structure design. Mater Today Phys 18:100394. https://doi.org/10.1016/j.mtphys.2021.100394
Rodrigues JEFS, Gainza J, Serrano-Sánchez F et al (2020) Structural features, anisotropic thermal expansion, and thermoelectric performance in bulk black phosphorus synthesized under high pressure. Inorg Chem 59:14932–14943. https://doi.org/10.1021/acs.inorgchem.0c01573
Zhao L-D, Lo S-H, Zhang Y et al (2014) Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 508:373–377. https://doi.org/10.1038/nature13184
Chang C, Wu M, He D et al (2018) 3D charge and 2D phonon transports leading to high out-of-plane ZT in n-type SnSe crystals. Science 360:778–783. https://doi.org/10.1126/science.aaq1479
Chang C, Tan G, He J et al (2018) The thermoelectric properties of SnSe continue to surprise: extraordinary electron and phonon transport. Chem Mater 30:7355–7367. https://doi.org/10.1021/acs.chemmater.8b03732
Chen ZG, Shi X, Zhao LD, Zou J (2018) High-performance SnSe thermoelectric materials: progress and future challenge. Prog Mater Sci 97:283–346. https://doi.org/10.1016/j.pmatsci.2018.04.005
Gu W-H, Zhang Y-X, Guo J et al (2021) Realizing high thermoelectric performance in n-type SnSe polycrystals via (Pb, Br) co-doping and multi-nanoprecipitates synergy. J Alloys Compd 864:158401. https://doi.org/10.1016/j.jallcom.2020.158401
Shi X, Tao X, Zou J, Chen Z (2020) High-performance thermoelectric SnSe: aqueous synthesis, innovations, and challenges. Adv Sci 1902923:1902923. https://doi.org/10.1002/advs.201902923
Rongione NA, Li M, Wu H et al (2019) High-performance solution-processable flexible SnSe nanosheet films for lower grade waste heat recovery. Adv Electron Mater. https://doi.org/10.1002/aelm.201800774
Horide T, Murakami Y, Hirayama Y et al (2019) Thermoelectric property in orthorhombic-domained SnSe film. ACS Appl Mater Interfaces 11:27057–27063. https://doi.org/10.1021/acsami.9b04868
Hou S, Li Z, Xue Y et al (2021) Surprisingly high in-plane thermoelectric performance in a-axis-oriented epitaxial SnSe thin films. Mater Today Phys 18:100399. https://doi.org/10.1016/j.mtphys.2021.100399
Bhattacharya M, Ranjan M, Kumar N, Maiti T (2021) Performance analysis and optimization of a SnSe-based thermoelectric generator. ACS Appl Energy Mater 4:8211–8219. https://doi.org/10.1021/acsaem.1c01466
Sraitrova K, Cizek J, Holy V et al (2019) Vacancies in SnSe single crystals in a near-equilibrium state. Phys Rev B 99:035306. https://doi.org/10.1103/PhysRevB.99.035306
Gowthamaraju S, Deshpande UP, Anwar S et al (2021) Effect of vacancy on thermoelectric properties of polycrystalline SnSe. J Mater Sci Mater Electron 32:11568–11576. https://doi.org/10.1007/s10854-021-05750-8
Liu M, Zhang J, Xu J et al (2020) The crystallization, thermodynamic and thermoelectric properties of vast off-stoichiometric Sn–Se crystals. J Mater Chem C 8:6422–6434. https://doi.org/10.1039/C9TC06903H
Liu M, Zhang J, Xu J et al (2020) Effect of Te doping on thermoelectric properties in vast off-stoichiometric tin-rich SnSe crystals. J Alloys Compd 836:155480. https://doi.org/10.1016/j.jallcom.2020.155480
Xie S, Cheng X, Hu C et al (2020) High pressure effect on the phase transition and in-plane anisotropy of SnSe. J Alloys Compd 849:155915. https://doi.org/10.1016/j.jallcom.2020.155915
Gharsallah M, Serrano-Sánchez F, Nemes NM et al (2016) Giant Seebeck effect in Ge-doped SnSe. Sci Rep 6:1–9. https://doi.org/10.1038/srep26774
Zhao Q, Qin B, Wang D et al (2020) Realizing high thermoelectric performance in polycrystalline SnSe via silver doping and germanium alloying. ACS Appl Energy Mater 3:2049–2054. https://doi.org/10.1021/acsaem.9b01475
Chandra S, Biswas K (2019) Realization of high thermoelectric figure of merit in solution synthesized 2D SnSe nanoplates via Ge alloying. J Am Chem Soc 141:6141–6145. https://doi.org/10.1021/jacs.9b01396
Gainza J, Serrano-Sánchez F, Gharsallah M et al (2019) Evidence of nanostructuring and reduced thermal conductivity in n-type Sb-alloyed SnSe thermoelectric polycrystals. J Appl Phys 126:045105. https://doi.org/10.1063/1.5108569
Prado-Gonjal J, Gainza J, Aguayo I et al (2020) High thermoelectric performance of rapidly microwave-synthesized Sn 1−δ S. Mater Adv 1:845–853. https://doi.org/10.1039/D0MA00301H
Gainza J, Serrano-Sánchez F, Gharsallah M et al (2018) Nanostructured thermoelectric chalcogenides. In: Aranguren P (ed) Bringing thermoelectricity into reality. InTech, pp 3–22
Serrano-Sánchez F, Nemes NM, Martínez JL et al (2018) Structural evolution of a Ge-substituted SnSe thermoelectric material with low thermal conductivity. J Appl Crystallogr 51:337–343. https://doi.org/10.1107/S1600576718000808
Gainza J, Serrano-Sánchez F, Rodrigues JEFS et al (2020) High-performance n-type SnSe thermoelectric polycrystal prepared by arc-melting. Cell Rep Phys Sci 1:100263. https://doi.org/10.1016/j.xcrp.2020.100263
Wei T-R, Tan G, Zhang X et al (2016) Distinct impact of alkali-ion doping on electrical transport properties of thermoelectric p-type polycrystalline SnSe. J Am Chem Soc 138:8875–8882. https://doi.org/10.1021/jacs.6b04181
Leng H-Q, Zhou M, Zhao J et al (2016) The thermoelectric performance of anisotropic SnSe doped with Na. RSC Adv 6:9112–9116. https://doi.org/10.1039/C5RA19469E
Ge Z-H, Song D, Chong X et al (2017) Boosting the thermoelectric performance of (Na, K)-codoped polycrystalline snse by synergistic tailoring of the band structure and atomic-scale defect phonon scattering. J Am Chem Soc 139:9714–9720. https://doi.org/10.1021/jacs.7b05339
Wei T-R, Wu C-F, Zhang X et al (2015) Thermoelectric transport properties of pristine and Na-doped SnSe 1–x Te x polycrystals. Phys Chem Chem Phys 17:30102–30109. https://doi.org/10.1039/C5CP05510E
Chen Y-X, Ge Z-H, Yin M et al (2016) Understanding of the extremely low thermal conductivity in high-performance polycrystalline SnSe through potassium doping. Adv Funct Mater 26:6836–6845. https://doi.org/10.1002/adfm.201602652
Zhang M, Wang D, Chang C et al (2019) Oxygen adsorption and its influence on the thermoelectric performance of polycrystalline SnSe. J Mater Chem C 7:10507–10513. https://doi.org/10.1039/C9TC03999F
Singh AK, Janotti A, Scheffler M, Van de Walle CG (2008) Sources of electrical conductivity in SnO2. Phys Rev Lett 101:055502. https://doi.org/10.1103/PhysRevLett.101.055502
Lee YK, Luo Z, Cho SP et al (2019) Surface oxide removal for polycrystalline SnSe reveals near-single-crystal thermoelectric performance. Joule 3:719–731. https://doi.org/10.1016/j.joule.2019.01.001
Byun J, An H, Hong J et al (2020) Thermoelectric performance of n-type polycrystalline SnSe with surface depletion by pressureless sintering. Appl Surf Sci 544:148834. https://doi.org/10.1016/j.apsusc.2020.148834
Li X, Li S, Li D et al (2021) Effect of hot pressing on the microstructure and thermoelectric properties of TGZM-grown YbFe-doped CoSb3 skutterudite. Ceram Int 47:8949–8958. https://doi.org/10.1016/j.ceramint.2020.12.017
Wang S, Hui S, Peng K et al (2017) Grain boundary scattering effects on mobilities in p-type polycrystalline SnSe. J Mater Chem C 5:10191–10200. https://doi.org/10.1039/C7TC03022C
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Phys B Phys Condens Matter 192:55–69. https://doi.org/10.1016/0921-4526(93)90108-I
Tang G, Wei W, Zhang J et al (2016) Realizing high figure of merit in phase-separated polycrystalline Sn 1–x Pb x Se. J Am Chem Soc 138:13647–13654. https://doi.org/10.1021/jacs.6b07010
Tang G, Wen Q, Yang T et al (2017) Rock-salt-type nanoprecipitates lead to high thermoelectric performance in undoped polycrystalline SnSe. RSC Adv 7:8258–8263. https://doi.org/10.1039/C7RA00140A
Chen CL, Wang H, Chen YY et al (2014) Thermoelectric properties of p-type polycrystalline SnSe doped with Ag. J Mater Chem A 2:11171–11176. https://doi.org/10.1039/c4ta01643b
Serrano-Sánchez F, Nemes NM, Dura OJ et al (2016) Structural phase transition in polycrystalline SnSe: a neutron diffraction study in correlation with thermoelectric properties. J Appl Crystallogr 49:2138–2144. https://doi.org/10.1107/S1600576716015405
Helmholz L (1936) The crystal structure of the low temperature modification of thallous iodide. Zeitschrift für Krist - Cryst Mater 95:129–137. https://doi.org/10.1524/zkri.1936.95.1.129
Jin M, Chen Z, Tan X et al (2018) Charge transport in thermoelectric SnSe single crystals. ACS Energy Lett 3:689–694. https://doi.org/10.1021/acsenergylett.7b01259
Zevalkink A, Smiadak DM, Blackburn JL et al (2018) A practical field guide to thermoelectrics: fundamentals, synthesis, and characterization. Appl Phys Rev 5:021303. https://doi.org/10.1063/1.5021094
May AF, Snyder GJ (2012) Introduction to modeling thermoelectric transport at high temperatures. In: Rowe DM (ed) Materials, preparation, and characterization in thermoelectrics. CRC Press, pp 1–18
Snyder GJ, Snyder AH, Wood M et al (2020) Weighted mobility. Adv Mater 32:2001537. https://doi.org/10.1002/adma.202001537
Zhang X, Bu Z, Shi X et al (2020) Electronic quality factor for thermoelectrics. Sci Adv 6:eabc0726. https://doi.org/10.1126/sciadv.abc0726
Acknowledgements
We are grateful to the Spanish Ministry of Science, Innovation and Universities (MICINN) for granting the projects MAT2017-84496-R and RTI2018-097895-B-C43. We thank ILL and ALBA personnel for making all facilities available for the neutron and synchrotron experiments. J.G. thanks MICINN for granting the contract PRE2018-083398.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
JG, JAA and NNM conceived and designed the study. JG and SM synthesized the samples. JG, JLM, NNM and OJD characterized the thermoelectric performance. JG, FS, JAA and MTFD carried out and analyzed the NPD data. NB carried and analyzed STEM/EELS measurements. JG and NNM coordinated the writing of the manuscript with discussion and input from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: M. Grant Norton.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gainza, J., Moltó, S., Serrano-Sánchez, F. et al. SnSe:Kx intermetallic thermoelectric polycrystals prepared by arc-melting. J Mater Sci 57, 8489–8503 (2022). https://doi.org/10.1007/s10853-021-06864-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06864-4