Abstract
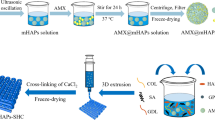
The prevention/treatment of osteomyelitis infection by combining the local antibiotic delivery system with the bone regeneration scaffold can effectively overcome the drawbacks of systemic antibiotic administration and realize the full cycle control of drug release. Herein, by introducing sodium carboxymethyl cellulose (SCMC) cross-linking agent to improve the binding force of poly(lactic-co-glycolic acid) (PLGA) microspheres and porous hydroxyapatite (HAp) bone scaffold. The porous HAp/PLGA drug-loaded microsphere bone scaffold for gentamicin sulfate (GS) delivery was successfully prepared. The optimal preparation parameters, drug release characteristics, SCMC enhancement mechanism, antibacterial properties and bone cell activity of porous HAp/PLGA drug-loaded microsphere bone scaffolds were comprehensively investigated. The results showed that the 0.1% SCMC-modified porous HAp/PLGA drug-loaded microsphere bone scaffold has a cumulative drug release of 45.0 ± 0.90% on the first day, which is about 20% lower than that of pure PLGA drug-loaded microspheres. Moreover, its drug release can be sustained and stably released for more than 17 d, which is attributed to the enhancement of the binding force between the microspheres and HAp by SCMC (combination for more than 3 weeks). Meanwhile, the diameter of the antibacterial ring expanded from the initial 10 ± 0.50 to 28 ± 1.2 mm after 14 d, which also indicated the sustained and stable release of GS. Alamar Blue analysis results showed that 0.1% SCMC-modified composite bone scaffold is beneficial to the proliferation activity of bone cells, and its 14 day activity increased by 20%. The above results indicate that the SCMC-modified composite bone scaffold has the potential to treat/prevent osteomyelitis.











Similar content being viewed by others
References
Muthukrishnan G, Masters EA, Daiss JL, Schwarz EM (2019) Mechanisms of immune evasion and bone tissue colonization that make S. aureus the primary pathogen in osteomyelitis. Curr Osteoporos Rep 17:395–404. https://doi.org/10.1007/s11914-019-00548-4
Zhang J, Wei Q, Zhou H et al (2020) Sustainable release of vancomycin from micro-arc oxidised 3D-printed porous Ti6Al4V for treating methicillin-resistant S. aureus bone infection and enhancing osteogenesis in a rabbit tibia osteomyelitis model. Biomater Sci 8:3106–3115. https://doi.org/10.1039/C9BM01968E
Mestres G, Fernandez-Yague MA, Pastorino D, Montufar EB, Canal C, Manzanares-Céspedes MC, Ginebra MP (2019) In vivo efficiency of antimicrobial inorganic bone grafts in osteomyelitis treatments. Mater Sci Eng C 97:84–95. https://doi.org/10.1016/j.msec.2018.11.064
Diefenbeck M, Schrader C, Gras F et al (2016) Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials 101:156–164. https://doi.org/10.1016/j.biomaterials.2016.05.039
Ryan EJ, Ryan AJ, González-Vázquez A et al (2019) Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 197:405–416. https://doi.org/10.1016/j.biomaterials.2019.01.031
Dhal C, Mishra R (2020) In vitro and in vivo evaluation of gentamicin sulphate-loaded PLGA nanoparticle-based film for the treatment of surgical site infection. Drug Deliv Transl Res 10:1032–1043. https://doi.org/10.1007/s13346-020-00730-7
Dorati R, DeTrizio A, Genta I, Grisoli P, Merelli A, Tomasi C, Conti B (2016) An experimental design approach to the preparation of pegylated polylactide-co-glicolide gentamicin loaded microparticles for local antibiotic delivery. Mater Sci Eng C 58:909–917. https://doi.org/10.1016/j.msec.2015.09.053
McKinney W, Yonovitz A, Smolensky MH (2015) Circadian variation of gentamicin toxicity in rats. Laryngoscope 125:252–256. https://doi.org/10.1002/lary.25116
Hahn H, Salt AN, Schumacher U, Plontke SK (2013) Gentamicin concentration gradients in scala tympani perilymph following systemic applications. Audiol Neurotol 18:383–391. https://doi.org/10.1159/000355283
Nandi SK, Bandyopadhyay S, Das P, Samanta I, Mukherjee P, Roy S, Kundu B (2016) Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol Adv 34:1305–1317. https://doi.org/10.1016/j.biotechadv.2016.09.005
Ji K, Wang YN, Wei QH, Zhang K, Jiang AG, Rao YW, Cai XX (2018) Application of 3D printing technology in bone tissue engineering. Bio-Design Manuf 1:203–210. https://doi.org/10.1007/s42242-018-0021-2
Kim H-W, Kim Y-J (2021) Fabrication of strontium-substituted hydroxyapatite scaffolds using 3D printing for enhanced bone regeneration. J Mater Sci 56:1673–1684. https://doi.org/10.1007/s10853-020-05391-y
Loca D, Sokolova M, Locs J, Smirnova A, Irbe Z (2015) Calcium phosphate bone cements for local vancomycin delivery. Mater Sci Eng C 49:106–113. https://doi.org/10.1016/j.msec.2014.12.075
Vorndran E, Geffers M, Ewald A, Lemm M, Gbureck U (2013) Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater 9:9558–9567. https://doi.org/10.1016/j.actbio.2013.08.009
Porta GD, Campardelli R, Cricchio V, Oliva F, Maffulli N, Reverchon E (2016) Injectable PLGA/Hydroxyapatite/Chitosan microcapsules produced by supercritical emulsion extraction technology: an in vitro study on teriparatide/gentamicin controlled release. J Pharm Sci 105:2164–2172. https://doi.org/10.1016/j.xphs.2016.05.002
Hamid ZAA, Tham CY, Ahmad Z (2018) Preparation and optimization of surface engineered poly(lactic acid) microspheres as a drug delivery device. J Mater Sci 53:4745–4758. https://doi.org/10.1007/s10853-017-1840-9
Bharadwaz A, Jayasuriya AC, Ambalangodage C (2020) Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C 110:110698. https://doi.org/10.1016/j.msec.2020.110698
Inzana JA, Schwarz EM, Kates SL, Awad HA (2016) Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 81:58–71. https://doi.org/10.1016/j.biomaterials.2015.12.012
Hu X, Shen H, Yang F, Liang XJ, Wang SG, Wu DC (2014) Modified composite microspheres of hydroxyapatite and poly(lactide-co-glycolide) as an injectable scaffold. Appl Surf Sci 292:764–772. https://doi.org/10.1016/j.apsusc.2013.12.045
Son JS, Appleford M, Ong JL, Wenke JC, Kim JM, Choi SH, Oh DS (2011) Porous hydroxyapatite scaffold with three-dimensional localized drug delivery system using biodegradable microspheres. J Control Release 153:133–140
Granado S, Cervera L, Kamen AA, Godia F (2018) Advancements in mammalian cell transient gene expression (TGE) technology for accelerated production of biologics. Crit Rev Biotechnol 38:918–940. https://doi.org/10.1080/07388551.2017.1419459
Lan WT, He L, Liu YW (2018) Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 8:291. https://doi.org/10.3390/coatings8080291
Qi P, Ohba S, Hara YC, Fuke M, Ogawa T, Ohta S, Ito T (2018) Fabrication of calcium phosphate-loaded carboxymethyl cellulose non-woven sheets for bone regeneration. Carbohyd Polym 189:322–330. https://doi.org/10.1016/j.carbpol.2018.02.050
Chittasupho C, Thongnopkoon T, Kewsuwan P (2016) Surface modification of poly(D, L-lactic-co-glycolic acid) nanoparticles using sodium carboxymethyl cellulose as colloidal stabilizer. Curr Drug Deliv 13:95–104. https://doi.org/10.2174/1567201812666150904144241
Locs J, Zalite V, Berzina-Cimdina L, Sokolova M (2013) Ammonium hydrogen carbonate provided viscous slurry foaming—A novel technology for the preparation of porous ceramics. J Eur Ceram Soc 33:3437–3443. https://doi.org/10.1016/j.jeurceramsoc.2013.06.010
Irbe Z, Loca D, Vempere D, Berzina-Cimdina L (2012) Controlled release of local anesthetic from calcium phosphate bone cements. Mater Sci Eng 32:1690–1694. https://doi.org/10.1016/j.msec.2012.04.069
Mao S, Xu J, Cai CF, Germershaus O, Schaper A, Kissel T (2007) Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharmaceut 334:137–148. https://doi.org/10.1016/j.ijpharm.2006.10.036
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–522. https://doi.org/10.1016/j.jconrel.2012.01.043
Chaisri W, Ghassemi AH, Hennink WE, Okonogi S (2011) Enhanced gentamicin loading and release of PLGA and PLHMGA microspheres by varying the formulation parameters. Colloid Surface B 84:508–514. https://doi.org/10.1016/j.colsurfb.2011.02.006
Ramazani F, Chen WL, van Nostrum CF, Storm G, Kiessling F, Lammers T, Hennink WE, Kok RJ (2016) Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: state-of-the-art and challenges. Int J Pharmaceut 499:358–367. https://doi.org/10.1016/j.ijpharm.2016.01.020
Sun S, Lin J, Zhang P, Fang L, Ma R, Quan ZG, Song X (2018) Geopolymer synthetized from sludge residue pretreated by the wet alkalinizing method: compressive strength and immobilization efficiency of heavy metal. Constr Build Mater 170:619–626. https://doi.org/10.1016/j.conbuildmat.2018.03.068
Dhal C, Mishra R (2019) Formulation development and in vitro evaluation of gentamicin sulfate-loaded PLGA nanoparticles based film for the treatment of surgical site infection by Box-Behnken design. Drug Dev Ind Pharm 45:805–818. https://doi.org/10.1080/03639045.2019.1576719
Cheng XK, He QJ, Li JQ, Huang ZL, Chi RA (2009) Control of pore size of the bubble-template porous carbonated hydroxyapatite microsphere by adjustable pressure. Cryst Growth Des 29:2770–2775. https://doi.org/10.1021/cg801421a
Flores C, Degoutin S, Chai F et al (2016) Gentamicin-loaded poly(lactic-co-glycolic acid) microparticles for the prevention of maxillofacial and orthopedic implant infections. Mater Sci Eng C 64:108–116. https://doi.org/10.1016/j.msec.2016.03.064
Van HN, Vu NH, Pham VH, Huan PV, Hoan BT, Nguyen DH, Manh TL (2020) A novel upconversion emission material based on Er3+–Yb3+–Mo6+ tridoped hydroxyapatite/tricalcium phosphate (HA/β-TCP). J Alloy Compd 827:154288. https://doi.org/10.1016/j.jallcom.2020.154288
Zhu TT, Cui YT, Zhang MG, Zhao DY, Liu GY, Ding JX (2020) Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact Mater 5:584–601. https://doi.org/10.1016/j.bioactmat.2020.04.008
Farhangdoust S, Zamanian A, Yasaei M, Khorami M (2013) The effect of processing parameters and solid concentration on the mechanical and microstructural properties of freeze-casted macroporous hydroxyapatite scaffolds. Mater Sci Eng C 33:453–460. https://doi.org/10.1016/j.msec.2012.09.013
Cui LG, Zhang J, Zou J et al (2020) Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials 230:119617. https://doi.org/10.1016/j.biomaterials.2019.119617
Lai YX, Cao HJ, Wang XL et al (2018) Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 153:1–13. https://doi.org/10.1016/j.biomaterials.2017.10.025
Wu CT, Ramaswamy Y, Zreiqat H (2010) Porous diopside (CaMgSi2O6) scaffold: a promising bioactive material for bone tissue engineering. Acta Biomater 6:2237–2245. https://doi.org/10.1016/j.actbio.2009.12.022
Zhao XY, Han Y, Zhu TT et al (2019) Electrospun polylactide-nano-hydroxyapatite-vancomycin composite scaffolds for advanced osteomyelitis therapy. J Biomed Nanotechnol 15:1213–1222. https://doi.org/10.1166/jbn.2019.2773
Li SQ, Dong SJ, Xu WG, Tu SC, Yan LS, Zhao CW, Ding JX, Chen XS (2018) Antibacterial Hydrogels. Adv Sci 5:1700527. https://doi.org/10.1002/advs.201700527
Dulon D, Aurousseau C, Erre JP, Aran JM (1988) Relationship between the nephrotoxicity and ototoxicity induced by gentamicin in the guinea pig. Acta Oto-Laryngol 106:219–225. https://doi.org/10.3109/00016488809106429
Posadowska U, Brzychczy-Włoch M, Drożdż A, Krok-Borkowicz M, Włodarczyk-Biegun M, Dobrzyński P, Chrzanowski W, Pamula E (2016) Injectable hybrid delivery system composed of gellan gum, nanoparticles and gentamicin for the localized treatment of bone infections. Expert Opin Drug Del 13:613–620. https://doi.org/10.1517/17425247.2016.1146673
Wang GH, Liu SJ, Ueng SWN, Chan EC (2004) The release of cefazolin and gentamicin from biodegradable PLA/PGA beads. Int J Pharmaceut 273:203–212. https://doi.org/10.1016/j.ijpharm.2004.01.010
Fredenberg S, Wahlgren M, Reslow M et al (2011) The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int J Pharmaceut 415:34–52. https://doi.org/10.1016/j.ijpharm.2011.05.049
Gasmi H, Siepmann F, Hamoudi MC, Axelsson A (2016) Towards a better understanding of the different release phases from PLGA microparticles: dexamethasone-loaded systems. Int J Pharmaceut 514:189–199. https://doi.org/10.1016/j.ijpharm.2016.08.032
Roth KE, Maier GS, Schmidtmann I, Eigner U, Huebner WD, Peters F, Drees P, Maus U (2019) Release of antibiotics out of a moldable collagen-beta-tricalciumphosphate-composite compared to two calcium phosphate granules. Materials 12:4056. https://doi.org/10.3390/ma12244056
Memar MY, Adibkia K, Farajnia S, Kafil HS, Dizaj SM, Ghotaslou R (2019) Biocompatibility, cytotoxicity and antimicrobial effects of gentamicin-loaded CaCO3 as a drug delivery to osteomyelitis. J Drug Deliv Sci Tec 54:101307. https://doi.org/10.1016/j.jddst.2019.101307
Choi CK, Kim H, Kwon H, Annable MD (2017) Effect of increased groundwater viscosity on the remedial performance of surfactant-enhanced air sparging. J Contam Hydrol 77:69–75. https://doi.org/10.1016/j.msec.2017.03.215
Zhao D, Zhu TT, Li J, Cui LG, Zhang ZY, Zhuang XL, Ding JX (2021) Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact Mater 6:346–360. https://doi.org/10.1016/j.bioactmat.2020.08.016
Szczeblinska J, Fijalkowski K, Kohn J, El Fray M (2017) Antibiotic loaded microspheres as antimicrobial delivery systems for medical applications. Mater Sci Eng C 77:69–75. https://doi.org/10.1016/j.msec.2017.03.215
Rostami F, Tamjid E, Behmanesh M (2020) Drug-eluting PCL/graphene oxide nanocomposite scaffolds for enhanced osteogenic differentiation of mesenchymal stem cells. Mater Sci Eng C 115:111102. https://doi.org/10.1016/j.msec.2020.111102
Zennifer A, Senthilvelan P, Sethuraman S, Sundaramurthi D (2021) Key advances of carboxymethyl cellulose in tissue engineering and 3D bioprinting applications. Carbohyd Polym 256:117561. https://doi.org/10.1016/j.carbpol.2020.117561
Chahal S, Hussain FSJ, Kumar A, Rasad MSBA, Yusoff MM (2016) Fabrication, characterization and in vitro biocompatibility of electrospun hydroxyethyl cellulose/poly (vinyl) alcohol nanofibrous composite biomaterial for bone tissue engineering. Chem Eng Sci 144:17–29. https://doi.org/10.1016/j.ces.2015.12.030
Acknowledgements
This work was supported by the China National Natural Science Foundation (No. 51878410), the China National Natural Science Foundation (No. 11672090) and the Shenzhen Science and Technology Planning Project (No. JCYJ20180507182310677).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Lisa White.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, X., Dai, L., Sun, S. et al. Preparation and performance of porous hydroxyapatite/poly(lactic-co-glycolic acid) drug-loaded microsphere scaffolds for gentamicin sulfate delivery. J Mater Sci 56, 15278–15298 (2021). https://doi.org/10.1007/s10853-021-06183-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06183-8