Abstract
In this work, we report that modification of the chemical composition of grain boundaries of La2/3-xLi3xTiO3 double perovskite, one of the most promising Li-ion conducting solid electrolytes, can be a convenient and versatile way of controlling the space charge potential, leading to a mitigated electrical resistance of the grain boundaries. Two groups of additives are investigated: lithium-enriching agents (Li3BO3, LiF) and 3d metal ions (Co2+, Cu2+), both expected to reduce the Schottky barrier. It is observed that Li-containing additives work effectively at a higher sintering temperature of 1250 °C. Regarding copper, it shows a much stronger positive impact at lower temperature, 1150 °C, while the addition of cobalt is always detrimental. Despite overall complex behavior, it is documented that the decreased space charge potential plays a more important role in the improvement of lithium conduction than the thickness of the grain boundaries. Among the proposed additives, modification of La2/3-xLi3xTiO3 by 2 mol.% Cu2+ results in the space charge potential reduction by 32 mV in relation to the reference sample, and the grain boundary specific conductivity increase by 80%, as measured at 30 °C. Introduced additive allows to obtain a similar effect on the conductivity as elevating the sintering temperature, which can facilitate manufacturing procedure.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to a rapidly increasing number of electric vehicles and a growing share of renewable energy sources in the power generation mix, the development of effective and reliable electrochemical energy storage systems has become very urgent. Among commercial reversible batteries, the highest performance is achieved for lithium-ion cells; however, it is believed that regrettably the Li-ion technology is close to the physical limits, and increasing safety requirements are not fully met. Solid-state lithium batteries, i.e., with the solid-state electrolyte, are considered to be a promising alternative for the state-of-the-art Li-ion batteries, as they would mitigate issues related to the presence of liquid organic electrolytes: flammability, chemical instability and risk of leakage, as well as possible dendritic growth of lithium during charging. In recent years, various organic materials, as well as inorganic materials, have been studied as solid electrolytes for reversible lithium cells [1,2,3,4]. Among them, La2/3-xLi3xTiO3 (LLTO) exhibits one of the highest bulk lithium ion conductivity, exceeding 1·10−3 S cm−1 at room temperature (RT) [5,6,7,8,9,10,11]. Taking into account its low electronic conductivity [6, 12], simple synthesis and good sinterability [5, 9, 13,14,15], as well as decent stability in air and at high redox potentials [13, 16], LLTO can be considered as a promising solid electrolyte material for solid-state lithium batteries.
LLTO is an A-site layered double perovskite with La-rich layers arranged alternately with Li-rich ones [12, 17]. As a consequence, such ordering results in a highly anisotropic, two-dimensional ionic transport [18]. Close investigations of the microstructure of polycrystalline sinters of LLTO revealed that besides grain boundaries, also high-angle domain boundaries present within crystallites exhibit reduced concentration of lithium [19,20,21,22]. Both features, 2D ionic transport and complex microstructure contribute to particularly low apparent grain boundary ionic conductivity (σgb,ap), about 10−7–10−6 S cm−1 [23,24,25,26] at RT, which is several orders of magnitude lower than the bulk ionic conductivity. It should be noticed, however, that there is a fundamental difference between the apparent and the specific conductivity of the grain boundaries (σgb). While the former is calculated using outer dimensions of the sample, the latter requires the determination of the microscopic size of the grain boundary, which is usually difficult to define. In this work, we apply the Brick Layer Model (BLM) [27] to calculate true specific grain boundary conductivity based on the ratio of bulk and grain boundary electrical capacitances measured using impedance spectroscopy technique (for details, see Supporting Information).
A few ways of enhancement of ionic conductivity in polycrystalline LLTO have been proposed. One of them is the coarsening of grains as a consequence of annealing, leading to a reduced fraction of boundaries in the sinter [15, 22]. It was reported that annealing of sinters of LLTO at temperatures between 1300 °C and 1400 °C caused a significant increase of the average size of domains and grains, resulting in enhanced apparent grain boundary conductivity, up to 3.55·10−4 S cm−1 at RT [5, 12, 15, 28,29,30,31]. Although this type of processing seems to give encouraging results, rising sintering temperature hampers technological applicability due to the increased costs and intensified lithium evaporation [5, 15], leading to difficulties in control of stoichiometry and lack of reproducibility.
Another proposed way to improve grain boundary conductivity is the alternation of its chemical composition. For example, Chen et al. [32] reported that introducing Li7La3Zr2O12 precursor sol into LLTO powder, and subsequent sintering at 1350 °C, changed grain boundary composition in such a way that σgb,ap raised from 0.63 ·10−4 S cm−1 to 1.5·10−4 S cm−1 at room temperature. Similarly as for high-temperature annealing, also here high-temperature heat treatment was required, which keeps technological limitations unresolved.
In fact, the issue of high resistance of grain boundaries is one of the major and ubiquitous concerns in the development of various solid electrolytes, e.g., Zr1-xYxO2 [33], Ce1-xGdxO2 [33], BaZr0.8Y0.2O3 [34], β-alumina [35, 36], NaSICONs [37,38,39], Li7La3Zr2O12 [40,41,42], which are important for numerous applications, such as fuel cells, batteries and electrolyzers.
According to a model developed by Guo and Maier for various ion-conducting oxides [27, 43], an electrostatic potential difference (Δφ), so-called the Schottky barrier or the space charge potential, is formed between a grain interior and a boundary core. Such potential difference affects the distribution of ionic and electronic charge carriers across the grain boundaries, and therefore, disturbs electrical conductivity at the grain boundary area. Wu and Guo applied this model for the LLTO electrolyte [22, 44], and confirmed quantitatively that the lithium-ion depletion zone at the grain boundary region was the actual reason of the high grain boundary resistance for Li+ transport. They also reported that an increase of the specific grain boundary conductivity can be achieved by chemical substitution of Ti with higher valence Nb in the La2/3-xLi3xTiO3 crystal. The formed bulk NbTi point defects, carrying a positive effective charge, allowed to decrease the Schottky barrier [44]. This can be interpreted as that more lithium is located at the grain boundaries. On the other hand, a similar effect can be obtained by the creation of point defects, e.g., MTi″, having a negative effective charge and being located at the grain boundary zone (Fig. 1). Through Coulombic interactions, such defects are expected to draw more lithium to the grain boundary zone. Alternatively, this can be also achieved by a simple, chemical enrichment of the boundaries with lithium excessive layer.
Consequently, in this work, we propose that modification of the chemical composition of grain boundaries, without changing the composition of the grain bulk, can be a convenient and versatile way of controlling the space charge potential, leading to mitigated resistance of the grain boundaries. Two groups of additives for La2/3-xLi3xTiO3 were selected: one comprises lithium-enriching agents (Li3BO3, LiF), aiming at a direct increase of concentration of lithium ions at the grain boundary region. The other one includes 3d metal ions (Co2+, Cu2+), affecting the Schottky barrier by the introduction of the charge lower than Ti4+, which is expected to indirectly, through electrostatic interactions, to rise the Li+ amount at the grain boundaries. Consequently, through such a modification, space charge potential is expected to be lowered. Besides this, the effect of the additives on sinterability and densification of the studied materials was investigated. It was documented that smaller space charge potential plays a more important role than the thickness of the grain boundaries. To the best of our knowledge, such studies are original and novel and the reported findings have not been published before. The results can be also regarded as having a broader impact, and be utilized in various solid electrolyte systems.
Materials and methods
Li0.33La0.56TiO3 perovskite was synthesized in a solid-state reaction between La2O3 (99.9%, Sigma Aldrich), TiO2 (anatase, 99.9%, Alfa Aesar), Li2CO3 (99%, Acros Organics). La2O3 before weighting was dried at 1000 °C for 12 h in a flow of dry synthetic air. All reactants were weighted in stoichiometric ratio and homogenized in a SPEX Sample Prep 8000 M high energy ball mill for 30 min in the 2-propanol medium. Homogenized reactants were pelletized to form 13 mm disk-shaped pellets and calcined at 1000 °C in dry synthetic air for 12 h. Then, calcined powders were ball-milled and uniaxially pressed under 150 MPa. Final synthesis and sintering were carried out at 1150 °C in dry synthetic air for 12 h. After sintering, LLTO disks were milled again to obtain fine LLTO powder. The distribution of particle size in the powder was measured with a Malvern Mastersizer 3000 particle size analyzer.
Such obtained LLTO powder was mixed with Li3BO3, LiF, Co(NO3)2 and Cu(NO3)2 additives in the amount of 2 mol.% in an agate mortar in methanol medium to form a homogenous paste. It is assumed that during heat treatment in the air in the subsequent steps the introduced nitrates decompose into corresponding oxides. Li3BO3 was synthesized in a solid-state reaction between Li2CO3 and B2O3 (anhydrous, 99.9%, Sigma Aldrich) at 600 °C for 10 h in synthetic airflow, according to the procedure described by Ohta et al. [40], while other additives were applied as purchased: Co(NO3)2·6H2O (99%, Sigma Aldrich), Cu(NO3)2·3H2O (99%, Sigma Aldrich), LiF (99%, Sigma Aldrich). After drying the paste at 70 °C, the obtained powder was formed into pellets, with the addition of 1 wt% of polyvinyl alcohol (Sigma Aldrich) to improve their strength. Then, the pellets were finally sintered at 1150 °C or 1250 °C for 12 h. The investigated samples were named according to Table 1.
Surface and cross-section microstructure was investigated using a scanning electron microscope FEI Nova Nano SEM 200 equipped with EDAX EDX elemental analyzer. Samples for surface analysis were polished on a fine sandpaper and thermally etched (1100 °C for 3 h in synthetic air), while pellets for cross-section investigations were immersed in epoxy resin, rubbed down with sandpaper, and polished using a diamond suspension.
Shrinkage curves during sintering were measured using Linseis L75HS 1600 horizontal dilatometer with a constant 5 ºC/min heating/cooling rate. The phase composition and unit cell parameters were examined by X-ray diffraction (XRD) method using CuKα radiation and a Panalytical Empyrean diffractometer equipped with a Pixcell 3D detector. Phase analysis of the XRD patterns was performed according to PDF-4 2018 database, and crystal lattice parameters were determined using the Rietveld refinement method as implemented in the GSAS/EXPGUI software.
Apparent density of the sintered pellets was measured using the hydrostatic method in a propanol environment according to PN/EN 1936:2001. The ionic conductivity of the sinters was investigated using the impedance spectroscopy technique. Prior to measurement, opposite surfaces of disk samples were covered with Au paste (ESL type 8844-G) and heated at 800 °C for 15 min to form well-defined continuous electrodes. Temperature dependence of the electrical conductivity of the samples was measured using a Solartron 1260 frequency response analyzer and a Probostat sample holder. Impedance spectra were recorded in a 1 MHz—1 Hz frequency range with sinusoidal excitation voltage of 0.1 V amplitude (r.m.s.), every 10 °C in 30—100 °C temperature range, in a dry air atmosphere. The frequency response was modeled using leastsquares fitting protocol as implemented in ZView2 software from Scribner Associates.
Analysis
Apparent macroscopic electrical conductivity of bulk- or grain boundaries in a polycrystalline sample is defined in Eq. 1:
where R is the electrical resistance for bulk or grain boundary, L is the distance between electrodes and A is their surface area.
Application of the BLM model [27] allows to determine the true specific grain boundary conductivity σgb, assuming that the effective thickness of a grain boundary δgb is related to its electrical capacitance according to Eq. 2.
where Cbulk and Cgb represent capacitance of grains and grain boundaries and d stands for average grain size, e.g., determined from electron microscopy images. As a constant phase element (CPE, impedance defined in Eq. 3) was used in the equivalent circuit applied to model impedance spectra the electrical capacitance, C, is estimated according to Eq. 4.
where: ZCPE—impedance of a Constant Phase Element, Q, n—parameters of the CPE, i—imaginary unit, ω—angular frequency of the AC signal.
Consequently, true specific grain boundary conductivity was calculated according to Eq. 5.
where
Once true specific conductivity for bulk and grain boundaries are known space charge potential (Schottky barrier) Δφ can be calculated from Eq. 7 [43].
where e, kB, z and T are the elementary electrical charge, Boltzmann constant, effective charge of the charge carriers and temperature in Kelvin scale.
Results
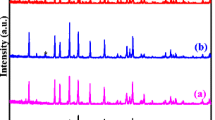
X-ray diffraction patterns (Fig. 2) revealed predominant tetragonal perovskite (P4/mmm) phase for all of the synthesized samples sintered both at 1150 °C and 1250 °C. For the reference LLTO samples sintered without additives all the diffraction peaks could be indexed with the perovskite crystal structure suggesting a single phase composition. On the other hand, for samples containing additives, new low-intensity peaks appeared, which were ascribed to secondary crystalline phases precipitated from the host material. Identified compounds, as well as their weight and molar fractions taken from Rietveld refinement, were gathered in Table 2.
To corroborate or refute the formation of solid solutions, unit cell parameters for the LLTO phase were calculated using Rietveld refinement of the XRD patterns for sample sintered with additives and compared with the unmodified reference samples. The obtained values were gathered in Table 3. The reference sample sintered at 1150 °C exhibited a = 3.8729 Å and c = 7.7504 Å. These values remain in good agreement with previously reported data for La2/3-xLi3xTiO3 perovskite with x equal to 0.11 [11], confirming the formation of the intended compound and indicating low lithium loss during sintering [45]. With sintering temperature increased to 1250 °C unit cell volume moderately expanded (+ 0.09%), suggesting slightly higher lithium evaporation.
Along with the effect that additives may have on transport properties of grain boundaries, also their effect on sintering should be considered. One of the key concerns, over the fabrication of LLTO electrolytes is lowering the sintering temperature. It was previously reported that high relative density and low porosity LLTO sinters can be fabricated via a sintering above 1300 °C [7, 8, 10, 12, 32]. In this work, however, we demonstrate that certain modifications of chemical composition can improve sinterability even at lower temperatures. In order to evaluate the effect of introduced additives on the sintering of LLTO powder, a dilatometric technique was applied. Relative shrinkage and shrinkage rate for the investigated samples as a function of temperature are presented in Fig. 3a, b. For the reference sample, a sintering curve with a single main shrinkage step was observed with an onset point at ca. 1165 °C and maximum sintering rate of 1.17% min−1 at 1230 °C. On the other hand, when LLTO powder was blended with the additives, the process of sintering can be markedly affected, and sintering temperature can be lowered. All temperatures for sintering onset and of maximum sintering rate were shown as an inset in Fig. 3. Above all, for the LBO sample, sintering onset was considerably reduced to 935 °C, while for other additives, sintering onset was between 1100 °C and 1140 °C. This can be connected with a low melting point of the additive (ca. 700 °C) and its glass forming ability. The addition of LiF, also having a low melting temperature of 850 °C, allows to decrease the onset temperature of sintering only by 50 °C, as compared with the reference sample. These observations were not investigated further as they were outside the main scope of this article; however, a tentative explanation of the observed difference between both liquid-phase forming additives can be related to the fact that they demonstrate different wettability of the LLTO grains. Similarly, relatively small effect on sintering of LLTO as for LiF was observed for Co2+ and Cu2+ containing additives. It is worth noting that with decreased sintering onset temperature sintering rate also decreased, probably due to a lower rate of mass transfer. Therefore, sintering with additives probably would require longer sintering times.
Figure 4 gathers scanning electron microscopy images presenting cross-sections of LLTO samples sintered with and without additives for 12 h at 1150 °C and 1250 °C. Exemplary images of surface microstructure of the reference, as well as the Cu_H sample, were presented in Fig. S1. Numerical average grain diameter along with the relative density of the sinters is gathered in Table 4.
The reference sample sintered at 1150 °C possessed well-developed grains with an average diameter close to 2.7 µm. In this case, a relatively large amount of porosity could be observed, which is consistent with a moderate relative density of about 83%. When sintering temperature was increased to 1250 °C, grains became larger (3.3 µm) and relative density reached 94%. Such high relative density led to negligible open porosity, as visible in SEM images. Among examined additives, Li3BO3, Co and Cu turned out to provide improved density after sintering at 1150 °C. The biggest improvement was observed for Co, where relative density was raised to 87% with unchanged sintering time. On the contrary, the introduction of LiF to LLTO electrolyte inhibited sintering at 1150 °C. When sintering temperature was elevated to 1250 °C, the effect of the additives became less visible, and regardless of the composition relative density was close to 94%. It is worth noting that the average grain size in the obtained sinters is relatively low and close to each other, as compared to other reports concerning LLTO [46, 47]. Furthermore, some cracks appeared at the surface of the Cu-modified sample (Fig. S2) sintered at 1250 °C, pointing to the build-up of stress, when this additive is used.
EDX analysis of chemical composition revealed considerable enrichment of grain boundary zone in copper for both, Cu_L and Cu_H samples. A line scan across a grain boundary of the Cu_H sample is presented in Fig. 5, whereas point spectra for Cu_L at several locations on the cross-section are shown in Fig. S2.
The concentration of copper in the grain boundaries reached 4.4 wt% (3.5 at.%) for Cu_L and 8.3 wt% (6.5 at.%) for Cu_H samples, whereas inside the grains it was below a limit of detection. These results indicate that the desired enrichment of the grain boundaries in copper was indeed achieved. However, ca. two times higher concentration of Cu at the grain boundaries of the Cu_H sample can be regarded as indicating increased aggregation of the modifier as a result of higher sintering temperature. This was also further confirmed as a relative number of the enriched places was lower in the Cu_H sample, despite the same used amount of the additive. Moreover, it was detected that some of the grain boundaries remained in their pristine state (see Fig. S2, point 1). Probably this is because the additives were introduced only to the outer surface of the powder particles, exposed during the impregnation step, while other grain boundaries, located inside the aggregate parties, remained unaffected. For all other samples, EDX spectra did not allow to unambiguously identify regions with enhanced levels of the introduced elements, neither as separate grains, nor as the continuous phase at grain boundaries. Nevertheless, the lack of any detectable regions enriched with Co (for Co_L and Co_H samples) suggests that cobalt rather is introduced as a bulk defect, likely taking place of titanium. This can be additionally supported by more similar radii of Ti4+ and Co2+ (in relation to Cu2+). For LBO and LF additives, EDX studies were inconclusive, due to low atomic numbers of lithium and boron, and small separation of the fluorine spectral line from the oxygen line which makes them difficult to analyze quantitatively.
When introducing 3d metal ions into the oxide matrix of a solid electrolyte, concerns often arise about the level of electronic component conductivity. In order to evaluate the effect of the introduced additives on ionic transference number, DC polarization measurements on sintered LLTO samples with Au electrodes were carried out. The obtained polarization curves are presented in Supplementary Information Fig. S3 and calculated ionic transference numbers are gathered in Table S1. Except for the Co_L sample, the ionic transference number did not drop below 0.98–0.99, indicating that the additive in the applied amount did not disturb the dominating ionic character of the electrical conductivity. Even for LLTO modified with Co (likely forming bulk defects), the ionic transference number remained above 0.9, pointing to the dominant ionic component of the charge transport.
Now, let us move to the main topic of this article, which is the evaluation of the true specific grain boundary conductivity and space charge potential of LLTO doped with Li3BO3, LiF, as well as Co2+ and Cu2+ ions. A technique allowing for insight into electrical properties of grain bulk and grain boundaries is impedance spectroscopy. Figure 6a, b shows Nyquist plots for the LLTO samples with additives. The observed spectra consisted of two semicircles located at high and medium frequency range, above 300 kHz and between 300 kHz and 100 Hz, respectively, and a linear part below 100 Hz. According to the standard interpretation of the impedance spectrum of polycrystalline solid electrolyte [48], the high-frequency semicircle is associated with the bulk ionic conductivity, while the medium frequency one originates from the grain boundary region; the linear part is related to the electrical response from the Au electrode. The spectra were modeled with the equivalent circuit used by Chen et al. [32] (Fig. 6c), where R1 and CPE1 describe bulk, R2 and CPE2 grain boundary, and CPE3 the electrode response. Next, corresponding specific bulk and specific grain boundary conductivities were calculated according to Eqs. 1–4, average grain sizes were determined from the SEM images as presented in Table 4. The obtained values of bulk and grain boundary specific conductivities for all the samples measured at 30 °C were gathered in Fig. 7.
Bulk ionic conductivity at 30 °C in the reference samples sintered without additives at 1150 °C and 1250 °C was determined to be 0.57 mS cm−1 and 1.1 mS cm−1 respectively, and the values are among the highest reported in the literature [8, 12, 32, 48]. In order to gain deeper insight into the observed changes in ionic conductivity of the LLTO samples, the activation energy of bulk and specific grain boundary components was calculated from their dependences on temperature. Corresponding Arrhenius plots are gathered in Supplementary Information Figs. S4 and S5, and the calculated activation energy values are presented in Table 5.
Discussion
As can be seen in Table 2, a small amount of precipitate was observed in the modified samples. However, as refined by the Rietveld method, the respective quantities of the secondary phases were considerably lower than the equivalent amount of the introduced additives. This indicates that the modifiers existed also beside the crystalline precipitates, forming either the desired enriched grain boundary zones, or (unwanted) solid solution with the LLTO perovskite. Furthermore, the obtained results showed that the higher sintering temperature generally favored a lower amount of the precipitated phases, plausibly indicating enhanced dissolving of the additives in the host LLTO crystallites as sintering temperature raised to 1250 °C. Two main trends could be observed for the samples sintered with additives. Firstly, when lithium-containing compounds were introduced, nearly zero changes of the unit cell volume took place versus the reference, which indicates that these additives prevent lithium loss and provide desirable simultaneous enrichment in lithium at grain boundaries. Secondly, samples containing Co2+ and Cu2+ additives exhibited a much more pronounced tendency of expansion of the unit cell volume. As no enrichment of grain boundary zone with Co was detected, for this material it could be explained by a partial substitution of Ti4+ with larger Co2+ cations. As a consequence of the electroneutrality condition, such bulk defects are expected to lead to an effective depletion of the grain boundaries with lithium, due to a reversed effect in relation to the one depicted in Fig. 1 i.e., formation of CoTi″ and an equivalent number of Lii· point defects in the grain bulk. This is expected not to be beneficial in terms of ionic transport at the grain boundary region.
In the case of the Cu-modified sample, lattice expansion can be regarded as being a result of the depletion of bulk LLTO with lithium ions. Here, grain boundaries were pronouncedly enriched with copper, as confirmed by SEM/EDX analysis (Figs. 5 and S2), which requires displacement of lithium ions from grain interior, in order to maintain electroneutrality. The existence of CuTi″ and in consequence, an equivalent number of Lii· point defects at the grain boundaries, should help to mitigate the blocking effect at the grain boundaries, as more lithium is located in this area (cf. Fig. 1).
Bulk lithium-ion conductivity
As for bulk ionic conductivity, it is important to emphasize that increased sintering temperature significantly improved the conduction, and what is remarkable, this effect could not be explained solely by the reduced porosity of the ceramic polycrystalline sinter. Further discussion in this work contributes to a better understanding of this observation. For samples sintered with additives at 1150 °C, bulk ionic conductivity remained virtually unaffected by the addition of Li3BO3, LiF and Co2+ ions, whereas Cu2+ caused its degradation to 0.33 mS cm−1. Such effect can be explained by lowered bulk lithium concentration inside grains of Cu-modified LLTO (as was explained before on a basis of electrostatic interactions of the defects). When sintering temperature increased to 1250 °C, Co2+ and Cu2+ additives diminished bulk conductivity in relation to the reference sample, whereas Li3BO3 and LiF additive resulted in unchanged bulk conductivity. Comparing to the lower sintering temperature, only Co-modified material shows different behavior, which is likely due to an increased amount of LiCo0.5Ti1.5O4 precipitate, also depleting bulk with lithium.
Grain boundary lithium-ion conductivity
Similarly, as in the case of bulk, increased sintering temperature improved also LLTO reference sample specific grain boundary conductivity, more than doubling its value from 1.2·10−8 S cm−1 to 3.1·10−8 S cm−1. To put the observed effects into a proper perspective, some of the studied additives introduced into LLTO sintered at 1150 °C allowed to obtain similar effect as elevating the sintering temperature (to 1250 °C), with the highest improvement in the grain boundary conductivity with the addition of Cu2+ and LiF, allowing to reach 2.1·10−8 S cm−1 and 1.7·10−8 S cm−1, respectively.
Another important issue is possible dependence or its lack of the specific grain boundary conductivity on the thickness of the grain boundaries (δgb). Fig. 8 shows the considered relationship calculated according to Eq. 2.
What could be noticed is that the temperature of sintering is a factor considerably affecting grain boundary thickness: δgb is around 15—35 nm for samples sintered at 1150 °C and around 10—15 nm for samples sintered at 1250 °C. Li3BO3 is the additive which reduces the grain boundary thickness, most notably at the lower sintering temperatures, whereas all the other investigated additives resulted in increased δgb. Remarkably, Cu_L sample possessed the highest specific grain boundary conductivity among all the samples sintered at 1150 °C, despite the thickest grain boundaries (ca. 20 nm). It seems that except for these observations, no further dependences could be deduced from this plot, which suggests that there should be another factor more strongly influencing specific grain boundary conductivity.
As previously discussed factors, such as the presence of the precipitated phases, changes of the unit cell parameters, porosity and grain size, could not convincingly explain the observed effect of additives on the electrical properties of grain boundaries, we kept looking for more suitable factors. Among them, the space charge potential proposed by Wu and Guo [22, 44] appears to be one of the key factors governing the concentration of lithium ion and therefore conductivity across the grain boundary region (cf. Eq. 7). Fig. 9 depicts the dependence of the grain boundary specific conductivity on the space charge potential for LLTO samples sintered with 2 mol.% additives at 1150 °C and 1250 °C.
Grain boundary specific conductivity measured at 30 °C as a function of space charge potential for Li0.33La0.56TiO3 samples with 2 mol.% additives sintered 1150 °C and 1250 °C. The dashed lines present relation between σgb and Δφ calculated directly from Eq. 7 for σbulk conductivity averaged for samples sintered at 1150 °C (dash line) and 1250 °C (dash-dot line)
The resulting dependence approximately follows the formula given in Eq. 7, as denoted by the dashed lines in Fig. 9, with samples having lower space charge potential exhibiting higher grain boundary specific conductivity for both sintering temperatures. Deviation from the theoretical behavior can be related to varying microstructure of the samples and other uncontrolled factors. At lower sintering temperature, the Cu and LiF modified samples exhibited lower space charge potential in comparison with the reference sample. As initially assumed, it indeed allowed to enhance Li+ conduction at grain boundaries (higher values of specific conductivity). In the case of the Cu_L material, the microscopic nature of this effect can be linked to the presence of CuTi″ substitution at the grain boundaries. According to the model described in the introduction, such modification leads to the raised lithium concentration at the grain boundaries. Nature of interactions of LiF with the grain boundaries could not be unequivocally explained, as its concentration in the grain boundaries was not confirmed by elemental analysis, however, possibly even a low concentration of Li+ and F− ions at the grain boundaries was sufficient to prevent lithium evaporation during sintering, and provide a reduction of the space charge potential. On the other hand, the presence of lithium and cobalt-containing secondary phase in the Co_L sample (Table 2) and simultaneous partial existence of CoTi″ defects in the bulk of LLTO crystallites remains in accordance with depleted grain boundaries in lithium. This results in raised Schottky potential and, as a consequence, decreased lithium ion conductivity. The unfavorable effect of LBO additive for the sample obtained at lower temperature likely stems from a presence of some amount of amorphous phase, not detectable on XRD (boron oxide is known as a glass-forming agent). Also, only in this case, the sintering curve (Fig. 3) indicated liquid phase-assisted sintering.
For the reference sample, increased sintering temperature led to decreased space charge potential, showing the beneficial effect at higher temperature. This can be explained by the increased grain size, similarly as it was observed by Wu and Guo [44]. On the contrary to the Cu-addition at 1150 °C, sintering of the Cu_H sample at 1250 °C did not allow to achieve enhancement of the σgb (Fig. 9). This can be explained by the discussed aggregation of Cu-containing precipitates, as evidenced in the EDX analysis, not fulfilling their role as initially expected. The other 3d-metal (cobalt) behaves the same at 1250 °C as at the lower sintering temperature. While the addition of LBO at higher temperatures did not allow to enhance grain boundary conductivity, LiF seems to be the only one effective modifier, likely due to its role as a lithium loss preventing agent.
Overall it can be stated that for enhancement of transport properties of LLTO, selection of the proper additives and the optimal (as low as possible) sintering temperature is crucial. Modification of the Schottky barrier is possible, but it is very sensitive to the conditions of the preparation route. Comparing Figs. 8 and 9, it can be stated that smaller Δφ is highly preferable, while the thickness of the grain boundaries plays a secondary role.
Noticeably, for all investigated samples, the activation energy for bulk conductivity remained approximately constant, irrespectively of sintering temperature and the additive, at a level of 0.33 ± 0.1 eV. This value is in good agreement with previous reports [22], and suggests that the additives hardly affected bulk migration enthalpy. Interestingly, for the modified samples, the activation energy value is not dependent directly on the sintering temperature, but it is lower for the material which shows lower Δφ (Table 5). This corresponds with the easier mobility of lithium ions crossing the grain boundary area if the Schottky barrier is decreased.
Conclusions
In this work, we discussed factors influencing grain boundary contribution to ionic conductivity in modified La2/3-xLi3xTiO3 lithium conducting solid electrolytes. It was shown that among multiple parameters, which have to be taken into account to achieve low grain boundary resistance, sintering temperature and modification of chemical composition plays a crucial role. A series of lithium- and 3d-metal-containing additives were investigated revealing that the most important mechanism linking the grain boundary ionic conductivity and properties of the additive is a modification of the Schottky potential at the grain boundaries. However, to obtain the desired effect for the particular additive, the sintering temperature must be optimized. Among the investigated additives, 2 mol.% Cu2+ for LLTO sintered at 1150 °C resulted in the space charge potential reduced by 32 mV in relation to the reference sample. As a result, the material showed grain boundary specific conductivity increased by 80%. It should be highlighted that for the optimized modifications, it is possible to obtain similar effects as elevating sintering temperature, which can be highly beneficial form the practical point of view.
References
Zhang Z, Shao Y, Lotsch B et al (2018) New horizons for inorganic solid state ion conductors. Energy Environ Sci 11:1945–1976. https://doi.org/10.1039/C8EE01053F
Zheng F, Kotobuki M, Song S et al (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 389:198–213. https://doi.org/10.1016/j.jpowsour.2018.04.022
Gao Z, Sun H, Fu L et al (2018) Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv Mater 30:1–27. https://doi.org/10.1002/adma.201705702
Sun C, Liu J, Gong Y et al (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386. https://doi.org/10.1016/j.nanoen.2017.01.028
Le HTT, Ngo DT, Kim Y-J et al (2017) A perovskite-structured aluminium-substituted lithium lanthanum titanate as a potential artificial solid-electrolyte interface for aqueous rechargeable lithium-metal-based batteries. Electrochim Acta 248:232–242. https://doi.org/10.1016/J.ELECTACTA.2017.07.110
Le HTT, Kalubarme RS, Ngo DT et al (2015) Citrate gel synthesis of aluminum-doped lithium lanthanum titanate solid electrolyte for application in organic-type lithium–oxygen batteries. J Power Sources 274:1188–1199. https://doi.org/10.1016/J.JPOWSOUR.2014.10.146
Inaguma Y, Chen L, Itoh M, Nakamura T (1994) Candidate compounds with perovskite structure for high lithium ionic conductivity. Solid State Ionics 70–71:196–202. https://doi.org/10.1016/0167-2738(94)90309-3
Lu D, Ma J, Wu J et al (2019) Preparation and electrochemical properties of Li0.33SrxLa0.56–2/3xTiO3-based solid-state ionic supercapacitor. Ceram Int 45:2584–2590. https://doi.org/10.1016/J.CERAMINT.2018.10.192
Yu K, Tian Y, Gu R et al (2018) Ionic conduction, colossal permittivity and dielectric relaxation behavior of solid electrolyte Li3xLa2/3-xTiO3 ceramics. J Eur Ceram Soc 38:4483–4487. https://doi.org/10.1016/J.JEURCERAMSOC.2018.05.023
Okumura T, Yokoo K, Fukutsuka T et al (2009) Improvement of Li-ion conductivity in a-site disordering lithium-lanthanum-titanate perovskite oxides by adding LiF in synthesis. J Power Sources 189:536–538. https://doi.org/10.1016/J.JPOWSOUR.2008.10.056
Stramare S, Thangadurai V, Weppner W (2003) Lithium lanthanum titanates: a review. Chem Mater 15:3974–3990. https://doi.org/10.1021/cm0300516
Bohnke O, Bohnke C, Fourquet JL (1996) Mechanism of ionic conduction and electrochemical intercalation of lithium into the perovskite lanthanum lithium titanate. Solid State Ionics 91:21–31. https://doi.org/10.1016/S0167-2738(96)00434-1
Chen CH, Amine K (2001) Ionic conductivity, lithium insertion and extraction of lanthanum lithium titanate. Solid State Ionics 144:51–57. https://doi.org/10.1016/S0167-2738(01)00884-0
Yu K, Gu R, Wu L et al (2018) Ionic and electronic conductivity of solid electrolyte Li0.5La0.5TiO3 doped with LiO2-SiO2-B2O3 glass. J Alloys Compd 739:892–896. https://doi.org/10.1016/J.JALLCOM.2017.12.361
Ban CW, Choi GM (2001) The effect of sintering on the grain boundary conductivity of lithium lanthanum titanates. Solid State Ionics 140:285–292. https://doi.org/10.1016/S0167-2738(01)00821-9
Zheng Z, Fang H-Z, Liu Z-K, Wang Y (2015) A fundamental stability study for amorphous LiLaTiO3 solid electrolyte. J Electrochem Soc 162:244–248. https://doi.org/10.1149/2.0011503jes
Fourquet JL, Duroy H, Crosnier-Lopez MP (1996) Structural and Microstructural Studies of the Series La2/3−xLi3x-1/3−2xTiO3. J Solid State Chem 127:283–294. https://doi.org/10.1006/jssc.1996.0385
Jay EE, Rushton MJD, Chroneos A et al (2015) Genetics of superionic conductivity in lithium lanthanum titanates. Phys Chem Chem Phys 17:178–183. https://doi.org/10.1039/C4CP04834B
Gao X, Fisher CAJ, Kimura T et al (2013) Lithium atom and a-site vacancy distributions in lanthanum lithium titanate. Chem Mater 25:1607–1614. https://doi.org/10.1021/cm3041357
Gao X, Fisher CAJ, Kimura T et al (2014) Domain boundary structures in lanthanum lithium titanates. J Mater Chem A 2:843–852. https://doi.org/10.1039/C3TA13726K
Ma C, Chen K, Liang C et al (2014) Atomic-scale origin of the large grain-boundary resistance in perovskite Li-ion-conducting solid electrolytes. Energy Environ Sci 7:1638. https://doi.org/10.1039/c4ee00382a
Wu J-F, Guo X (2017) Size effect in nanocrystalline lithium-ion conducting perovskite: Li0.30La0.57TiO3. Solid State Ionics 310:38–43. https://doi.org/10.1016/j.ssi.2017.08.003
Moriwake H, Gao X, Kuwabara A et al (2015) Domain boundaries and their influence on Li migration in solid-state electrolyte (La, Li)TiO3. J Power Sources 276:203–207. https://doi.org/10.1016/j.jpowsour.2014.11.139
Aguesse F, Roddatis V, Roqueta J et al (2015) Microstructure and ionic conductivity of LLTO thin films: influence of different substrates and excess lithium in the target. Solid State Ionics 272:1–8. https://doi.org/10.1016/j.ssi.2014.12.005
Wolfenstine J, Allen JL, Read J et al (2010) Hot-pressed Li0.33La0.57TiO3. J Power Sources 195:4124–4128. https://doi.org/10.1016/j.jpowsour.2009.12.109
Geng HX, Mei A, Dong C et al (2009) Investigation of structure and electrical properties of Li0.5La0.5TiO3 ceramics via microwave sintering. J Alloys Compd 481:555–558. https://doi.org/10.1016/j.jallcom.2009.03.038
Guo X, Maier J (2001) Grain boundary blocking effect in zirconia: a schottky barrier analysis. J Electrochem Soc 148:E121–E126
Geng H, Mei A, Lin Y, Nan C (2009) Effect of sintering atmosphere on ionic conduction and structure of Li0.5La0.5TiO3 solid electrolytes. Mater Sci Eng B 164:91–95. https://doi.org/10.1016/J.MSEB.2009.07.011
Inaguma Y, Nakashima M (2013) A rechargeable lithium-air battery using a lithium ion-conducting lanthanum lithium titanate ceramics as an electrolyte separator. J Power Sources 228:250–255. https://doi.org/10.1016/j.jpowsour.2012.11.098
Sotomayor ME, Várez A, Bucheli W et al (2013) Structural characterisation and Li conductivity of Li1/2−xSr2xLa1/2−xTiO3 (0<x<0.5) perovskites. Ceram Int 39:9619–9626. https://doi.org/10.1016/j.ceramint.2013.05.083
Kwon WJ, Kim H, Jung K-N et al (2017) Enhanced Li+ conduction in perovskite Li3xLa2/3−x-1/3−2xTiO3 solid-electrolytes via microstructural engineering. J Mater Chem A 5:6257–6262. https://doi.org/10.1039/C7TA00196G
Chen K, Huang M, Shen Y et al (2013) Improving ionic conductivity of Li0.35La0.55TiO3 ceramics by introducing Li7La3Zr2O12 sol into the precursor powder. Solid State Ionics 235:8–13. https://doi.org/10.1016/j.ssi.2013.01.007
GerhardtNOWICK RAS (1986) Grain-boundary effect in ceria doped with trivalent cations: i, electrical measurements. J Am Ceram Soc 69:641–646. https://doi.org/10.1111/j.1151-2916.1986.tb07464.x
Sun Z, Fabbri E, Bi L, Traversa E (2011) Lowering grain boundary resistance of BaZr0.8Y0.2O3−δ with LiNO3 sintering-aid improves proton conductivity for fuel cell operation. Phys Chem Chem Phys 13:7692–7700. https://doi.org/10.1039/C0CP01470B
Zhu C, Xue J, Ji G (2015) Effect of Na2O content on properties of beta alumina solid electrolytes. Mater Sci Semicond Process 31:487–492. https://doi.org/10.1016/J.MSSP.2014.12.038
Lu X, Xia G, Lemmon JP, Yang Z (2010) Advanced materials for sodium-beta alumina batteries: Status, challenges and perspectives. J Power Sources 195:2431–2442. https://doi.org/10.1016/J.JPOWSOUR.2009.11.120
Lunghammer S, Ma Q, Rettenwander D et al (2018) Bulk and grain-boundary ionic conductivity in sodium zirconophosphosilicate Na3Zr2(SiO4)2PO4 (NASICON). Chem Phys Lett 701:147–150. https://doi.org/10.1016/J.CPLETT.2018.04.037
Zając W, Tarach M, Trenczek-Zając A (2017) Towards control over redox behaviour and ionic conductivity in LiTi2(PO4)3 fast lithium-ion conductor. Acta Mater 140:417–423. https://doi.org/10.1016/j.actamat.2017.08.064
Shao Y, Zhong G, Lu Y et al (2019) A novel NASICON-based glass-ceramic composite electrolyte with enhanced Na-ion conductivity. Energy Storage Mater 23:514–521. https://doi.org/10.1016/j.ensm.2019.04.009
Ohta S, Komagata S, Seki J et al (2013) All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J Power Sources 238:53–56. https://doi.org/10.1016/J.JPOWSOUR.2013.02.073
Ohta S, Kihira Y, Asaoka T (2016) Grain boundary analysis of the garnet-like oxides Li7+X−YLa3−XAXZr2−YNbYO12 (A = Sr or Ca). Front Energy Res 4:30. https://doi.org/10.3389/fenrg.2016.00030
Yu S, Siegel DJ (2017) Grain boundary contributions to li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO). Chem Mater 29:9639–9647. https://doi.org/10.1021/acs.chemmater.7b02805
Guo X, Sigle W, Maier J (2003) Blocking grain boundaries in yttria-doped and undoped ceria ceramics of high purity. J Am Ceram Soc 86:77–87. https://doi.org/10.1111/j.1151-2916.2003.tb03281.x
Wu J-F, Guo X (2017) Origin of the low grain boundary conductivity in lithium ion conducting perovskites: Li3xLa0.67−xTiO3. Phys Chem Chem Phys 19:5880–5887. https://doi.org/10.1039/C6CP07757A
Harada Y, Ishigaki T, Kawai H, Kuwano J (1998) Lithium ion conductivity of polycrystalline perovskite La0.67-xLi3xTiO3 with ordered and disordered arrangements of the A-site ions. Solid State Ionics 108:407–413. https://doi.org/10.1016/s0167-2738(98)00070-8
Jiang Z, Xie H, Wang S et al (2018) Perovskite membranes with vertically aligned microchannels for all-solid-state lithium batteries. Adv Energy Mater 8:1–7. https://doi.org/10.1002/aenm.201801433
Jiang Z, Wang S, Chen X et al (2020) Tape-casting Li0.34La0.56TiO3 ceramic electrolyte films permit high energy density of lithium-metal batteries. Adv Mater 32:1–7. https://doi.org/10.1002/adma.201906221
Inaguma Y, Liquan C, Itoh M et al (1993) High ionic conductivity in lithium lanthanum titanate. Solid State Commun 86:689–693. https://doi.org/10.1016/0038-1098(93)90841-A
Acknowledgements
This work was funded by the National Science Centre of Poland as part of the Grant No. 2012/05/D/ST5/00472. This work was carried out using the infrastructure of Laboratory of Materials for Energy Conversion and Storage at the Centre of Energy, AGH University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to declare.
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Polczyk, T., Zając, W., Ziąbka, M. et al. Mitigation of grain boundary resistance in La2/3-xLi3xTiO3 perovskite as an electrolyte for solid-state Li-ion batteries. J Mater Sci 56, 2435–2450 (2021). https://doi.org/10.1007/s10853-020-05342-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05342-7