Abstract
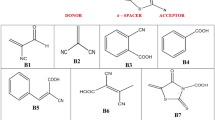
A simple and rapid synthesis of six new, stable β-ketoenamines by coupling 3-amino-1,8-naphthalimide derivatives with 2-(4-pyrimidinyl)-malondialdehyde in the presence of trifluoroacetic acid was developed. Two of the synthesized 3-amino-1,8-naphthalimide derivatives were condensed with 9H-fluorene-2-carboxaldehyde, resulting in azomethine products. The structure of obtained compounds was confirmed by 1H and 13C NMR, COSY, HMQC, FTIR spectroscopy and elemental analysis. The photophysical, electrochemical and thermal properties of the compounds were investigated. Additionally, calculations using density functional theory were performed to obtain the optimized ground-state geometry and distribution of the HOMO and LUMO levels as well as UV–Vis and photoluminescence spectra of the synthesized compounds. DSC measurements revealed that the compounds form amorphous material with glass transition temperature in the range of 41–146 °C and are thermally stable up to 300 °C. β-Ketoenamines have higher glass transition temperature, smaller energy gap (2.11–2.47 eV), higher PL quantum efficiency in solution (5.25–12.97%) and ability to aggregation-induced emission than the azomethines.








Similar content being viewed by others
References
Do TT, Takeda Y, Manzhos S, Bell J, Tokito S, Sonar P (2018) Naphthalimide end capped anthraquinone based solution-processable n-channel organic semiconductors: effect of alkyl chain engineering on charge transport. J Mater Chem C 6:3774–3786
Sonalin S, Sakthivel K, Nagarajan S (2018) Functionalization of 1, 8-naphthalimides—an approach towards air-stable n-type organic semiconductors. Mater Today Proc 5:16592–16597. https://doi.org/10.1016/j.matpr.2018.06.016
Kolosov D, Adamovich V, Djurovich P, Thompson ME, Adachi Ch (2002) 1,8-Naphthalimides in phosphorescent organic LEDs: the interplay between dopant, exciplex, and host emission. J Am Chem Soc 124:9945–9954
Zeng W, Lai HY, Lee WK, Jiao M, Shiu YJ, Zhong Ch, Gong S, Zhou T, Xie G, Sarma M, Wong KT, Wu ChCh, Yang Ch (2018) Achieving nearly 30% external quantum efficiency for orange-red organic light emitting diodes by employing thermally activated delayed fluorescence emitters composed of 1,8-naphthalimide-acridine hybrids. Adv Mater 30:1704961
Schab-Balcerzak E, Siwy M, Filapek M, Kula S, Malecki JG, Laba K, Lapkowski M, Janeczek H, Domanski M (2015) New core-substituted with electron-donating group 1,8-naphthalimides towards optoelectronic applications. J Lumin 166:22–39
Kotowicz S, Korzec M, Siwy M, Golba S, Małecki JG, Janeczek H, Maćkowski S, Bednarczyk K, Libera M, Schab-Balcerzak E (2018) Novel 1,8-naphthalimides substituted at 3-C position: synthesis and evaluation of thermal, electrochemical and luminescent properties. Dyes Pigm 158:65–78
Zhang J, Xiao H, Zhang X, Wu Y, Li G, Li C, Chen X, Ma W, Bo Z (2016) 1,8-Naphthalimide-based nonfullerene acceptors for wide optical band gap polymer solar cells with an ultrathin active layer thickness of 35 nm. J Mater Chem C 4:5656–5663
Gautam P, Sharma R, Misra R, Keshtov ML, Kuklin SA, Sharma GD (2017) Donor–acceptor–acceptor (D–A–A) type 1,8-naphthalimides as non-fullerene small molecule acceptors for bulk heterojunction solar cells. Chem Sci 8:2017–2024
Zhang J, Zhang X, Xiao H, Li G, Liu Y, Li C, Huang H, Chen X, Bo Z (2016) 1,8-naphthalimide-based planar small molecular acceptor for organic solar cells. ACS Appl Mater Interfaces 8:5475–5483
Zhu M, Miao J, Hu Z, Chen Y, Liu M, Murtaza I, Meng H (2017) A novel A–D–A small molecule with 1,8-naphthalimide as a potential non-fullerene acceptor for solution processable solar cells. Dyes Pigm 142:39–50
Banerjee S, Veale EB, Phelan CM, Murphy SA, Tocci GM, Gillespie LJ, Frimannsson DO, Kelly JM, Gunnlaugsson T (2013) Recent advances in the development of 1,8-naphthalimide based DNA targeting binders anticancer and fluorescent cellular imaging agents. Chem Soc Rev 42:1601–1618
Wang L, Fujii M, Yamaji M, Okamoto H (2018) Fluorescence behaviour of 2-, 3- and 4-amino-1,8-naphthalimides: effects of the substitution positions of the amino functionality on the photophysical properties. Photochem Photobiol Sci 17:1319–1328
Gopikrishna P, Meher N, Iyer PK (2018) Functional 1,8-naphthalimide AIE/AIEEgens: recent advances and prospects. ACS Appl Mater Interfaces 10:12081–12111
Quaquebeke EV, Mahieu T, Dumont P, Dewelle J, Ribaucour F, Simon G, Sauvage S, Gaussin JF, Tuti J, Yazidi ME, Vynckt FV, Mijatovic T, Lefranc F, Darro F, Kiss R (2007) 2,2,2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin 5-yl}carbamoyl)acetamide (UNBS3157), a novel nonhematotoxic naphthalimide derivative with potent antitumor activity. J Med Chem 50:4122–4134
Kascheres CM (2003) The chemistry of enaminones, diazocarbonyls and small rings: our contribution. J Braz Chem Soc 6:945–969
Romero-Fernández MP, Ávalos M, Babiano R, Cintas P, Jiménez JL, Light ME, Palaciosa JC (2014) Pseudo-cyclic structures of mono- and di-azaderivatives of malondialdehydes Synthesis and conformational disentanglement by computational analyses. Org Biomol Chem 12:8997–9010
Singh V, Jang S, Vishwakarma NK, Kim DP (2018) Intensified synthesis and post-synthetic modification of covalent organic frameworks using a continuous flow of microdroplets technique. NPG Asia Mater 10:e456. https://doi.org/10.1038/am.2017.209
Kong W, Wan J, Namuangruk S, Guo J, Wang Ch (2018) Water-soluble metalated covalent organic nanobelts with improved bioavailability for protein transportation. Sci Rep 8:5529. https://doi.org/10.1038/s41598-018-23744-1
Daugherty MC, Vitaku E, Li RL, Evans AM, Chavez AD, Dichtel WR (2019) Improved synthesis of b-ketoenamine-linked covalent organic frameworks via monomer exchange reactions. ChemComm 55:2680–2683
Vitaku E, Dichtel WR (2017) Synthesis of 2D imine-linked covalent organic frameworks through formal transimination reactions. J Am Chem Soc 139:12911–12914
Chaia S, Liu H, Zhang X, Han Y, Hu N, Wei L, Cong F, Wei H, Wang L (2016) Synthesis of a novel β-ketoenamine-linked conjugated microporous polymer with NH functionalized pore surface for carbon dioxide capture. Appl Surf Sci 384:539–543
Sedgwick AC, Wu L, Han HH, Bull SD, He HP, James TD, Sessler JL, Tang BZ, Tian H, Yoon J (2018) Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem Soc Rev 47:8842–8880
Olyaei A, Javarsineh S, Sadeghpour M (2018) Green synthesis and Z/E-isomerization of novel coumarin enamines induced by organic solvents. Chem Heterocycl Compd 54:934–939
Deng D, Xiao L, Chung IM, Kim IS, Gopiraman M (2017) Industrial-quality graphene oxide switched highly efficient metal and solvent-free synthesis of β-ketoenamines under feasible conditions. ACS Sustain Chem Eng 5:253–1259
Liu JY, Cao GE, Xu W, Cao J, Wang WL (2010) Ni(OAc)2: a highly efficient catalyst for the synthesis of enaminone and enamino ester derivatives under solvent-free conditions. Appl Organomet Chem 24:685–691
Martınez RF, Avalos M, Babiano R, Cintas P, Jimenez JL, Lightb ME, Palacios JC (2011) Tautomerism in Schiff bases. The cases of 2-hydroxy-1-naphthaldehyde and 1-hydroxy-2-naphthaldehyde investigated in solution and the solid state. Org Biomol Chem 9:8268–8275
Rauf MA, Hisaindee S, Saleh N (2015) Spectroscopic studies of keto–enol tautomeric equilibrium of azo dyes. RSC Adv 5:18097–18110
Qina X, Dinga G, Wanga Z, Zhanga S, Lia H, Luoa Z, Gaoa F (2017) Remarkable difference between five- and six- number-membered ring transition states for intramolecular proton transfer in excited state. J Photochem Photobiol, A 330:25–35
Afonin AV, Pavlov DV, Vashchenko AV (2019) Case study of 2-vinyloxypyridine: quantitative assessment of the intramolecular CeH/N hydrogen bond energy and its contribution to the one-bond 13Ce1. J Mol Struct 1176:73–85
Shirota Y (2005) Photo- and electroactive amorphous molecular materials—molecular design, syntheses, reactions, properties, and applications. J Mater Chem 15:75–93
Shitota Y, Kageyama H (2007) Charge carrier transporting molecular materials and their applications in devices. Chem Rev 107:953–1010
Yin SW, Shuai Z, Wang Y (2003) A quantitative structure-property relationship study of the glass transition temperature of OLED materials. J Chem Inf Comput Sci 43:970–977
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Nakatsuji J, Cheeseman R, Scalmani G, Barone V, Mennucci B, Petersson GA, Caricato HM, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehar M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2016) Gaussian 09, Revision D.01. Gaussian Inc, Wallingford
O’boyle NM, Tenderholt, Langner KM, Langner KM (2008) cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Barone V, Cossi M (1997) A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J Chem Phys 107:3210–3221
Yadav BS, Singh V (1999) Spectral investigations and thermodynamic functions of 4-hydroxy-2-mercapto-6-propylpyrimidine. Spectrochim Acta, Part A 55:1267–1278
Asiri AM, Badahdah KO (2007) Synthesis of some new anils: part 1. Reaction of 2-hydroxy-benzaldehyde and 2-hydroxynaphthaldehyde with 2-aminopyridene and 2-aminopyrazine. Molecules 12:1796–1804
Zutterman F, Louant O, Mercier G, Leyssens T, Champagne B (2018) Predicting keto − enol equilibrium from combining UV/visible absorption spectroscopy with quantum chemical calculations of vibronic structures for many excited states. A case study on salicylideneanilines. J Phys Chem A 122:5370–5374
Runge E, Gross EKU (1984) Density-functional theory for time-dependent systems. Phys Rev Lett 52:997–1000
Takagi K, Yamada Y, Fukuda R, Ehara M, Takeuchi D (2018) ESIPT emission behavior of methoxy-substituted 2-hydroxyphenylbenzimidazole isomers. New J Chem 42:5923–5928
Padalkar VS, Sakamaki D, Kuwada K, Tohnai N, Akutagawa T, Sakaid K, Seki S (2016) AIE active triphenylamine–benzothiazole based motifs: ESIPT or ICT emission. RSC Adv 6:26941–26949
Qina X, Dinga G, Wanga Z, Zhanga S, Lia H, Luoa Z, Gaoa F (2017) Remarkable difference between five- and six- number-membered ring transition states for intramolecular proton transfer in excited state. J Photochem Photobiol A Chem 339:25–35
Li Ch, Li D, Shi Y, Liu Y (2018) Excited-state intramolecular proton transfer in non-fused five- and fused six-membered ring pyrrole-pyridine hydrogen bond systems. Org Electron 54:177–183
Padalkar VS, Seki S (2016) Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem Soc Rev 45:169–202
Kenfack CA, Klymchenko AS, Duportail G, Burgerc A, Melya Y (2012) Ab initio study of the solvent H-bonding effect on ESIPT reaction and electronic transitions of 3-hydroxychromone derivatives. Phys Chem Chem Phys 14:8910–8918
Wiethaus G, Toldo JM, Santos FS, Duarte RC, Gonçalves PFB, Rodembusch FS (2019) Experimental and theoretical investigation of long-wavelength fluorescence emission in push–pull benzazoles: intramolecular proton transfer or charge transfer in the excited state? Phys Chem Chem Phys 21:4408–4420
Georgiev NI, Krasteva PV, Bojinov VB (2019) A ratiometric 4-amido-1,8-naphthalimide fluorescent probe based on excimer-monomer emission for determination of pH and water content in organic solvents. J Lumin 212:271–278
Uhler B, Ivanov MV, Kokkin D, Reilly N, Rathore R, Reid SA (2017) Effect of facial encumbrance on excimer formation and charge resonance stabilization in model bichromophoric assemblies. J Phys Chem C 121:15580–15588
Mai S, Ashwood B, Marquet P, Crespo-Hernandez CE, Gonzalez L (2017) Solvatochromic effects on the absorption spectrum of 2-Thiocytosine. J Phys Chem B 121:5187–5196
Chipem FAS, Mishra A, Krishnamoorthy G (2012) The role of hydrogen bonding in excited state intramolecular charge transfer. Phys Chem Chem Phys 14:8775–8790
Li H, Ma L, Yin H, Shi Y (2018) Effect of intramolecular and intermolecular hydrogen bonding on the ESIPT process in DEAHB molecule. Chin Phys B 9:1–6
Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ (2015) Aggregation-induced emission: together we shine, united we soar! Chem Rev 115:11718–11940
Hong Y, Lama JWY, Tang BZ (2009) Aggregation-induced emission: phenomenon, mechanism and applications. Chem Commun 29:4332–4353. https://doi.org/10.1039/b904665h
Chen Y, Lam JWY, Kwok RTK, Liu B, Tang BZ (2019) Aggregation-induced emission: fundamental understanding and future developments. Mater Horiz 6:428–433
Xue P, Sun J, Chen P, Gong P, Yao B, Zhang Z, Qiana Ch, Lu R (2015) Strong solid emission and mechanofluorochromism of carbazole-based terephthalate derivatives adjusted by alkyl chains. J Mater Chem C 3:4086–4092
Zhu X, Liu R, Li Y, Huang H, Wang Q, Wang D, Zhu X, Liuc S, Zhu H (2014) An AIE-active boron-difluoride complex: multi-stimuli-responsive fluorescence and application in data security protection. Chem Commun 50:12951–12954
Gan X, Liu G, Chu M, Xi W, Ren Z, Zhang X, Tian Y, Zhou H (2017) Intermolecular interactions boost aggregation induced emission in carbazole Schiff base derivatives. Org Biomol Chem 15:256–264
Pramanik B, Das D (2018) Aggregation-induced emission or hydrolysis by water? The case of Schiff bases in aqueous organic solvents. J Phys Chem C 122:3655–3661
Acknowledgements
Calculations have been carried out using resources provided by Wroclaw Centre for Networking and Supercomputing (http://wcss.pl), Grant No. 18.
Author information
Authors and Affiliations
Contributions
MK conceived and designed the experiments. MK, SK, RRK and MC performed the experiments. MK, ESB, JGM and MC analyzed the data. MK, ESB, JGM and MŁ wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Korzec, M., Kotowicz, S., Rzycka-Korzec, R. et al. Novel β-ketoenamines versus azomethines for organic electronics: characterization of optical and electrochemical properties supported by theoretical studies. J Mater Sci 55, 3812–3832 (2020). https://doi.org/10.1007/s10853-019-04210-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04210-3