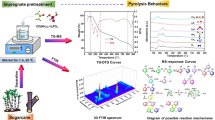
Abstract
Presented herein is the nitrogen-doped multiwalled carbon nanotubes (N-MWCNTs) production from a residue, sugarcane bagasse, using 1-butyl-3-methylimidazolium chloride [C4MIM]Cl as the solvent and nitrogen source, and ferrocene as the catalyst source. N-MWCNTs were synthesised using the floating catalyst chemical vapour deposition method at 850 °C. The synthesised N-MWCNTs were characterised using transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), thermogravimetric analysis (TGA), X-ray diffraction (XRD) spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and Raman spectroscopy. Hollow tubular structures of N-MWCNTs were observed using TEM. These observations correlated morphology from SEM which showed spaghetti-like structures, and also EDS detected the presence of nitrogen. Raman spectroscopy indicated MWCNT bands, around 1350 and 1580 cm−1 assigned to D-band and G-band due to defective and graphitic carbon vibrations, respectively. Also, XRD patterns showed typical N-MWCNT structures with a strong intensity peak at 2θ = 26.4° which was indexed as the C(002) reflection of graphite. TGA showed an N-CNTs thermogram curve, with the main decomposition temperature around 590 °C. The study showed that N-MWCNTs were successfully synthesised from sugarcane bagasse. The study significantly establishes a strategy for utilisation and value addition of a residue which is abundant from sugar production mills.








Similar content being viewed by others
References
Suganya T, Varman M, Masjuki HH, Renganathan S (2016) Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: a biorefinery approach. Renew Sustain Energy Rev 55:909–941. https://doi.org/10.1016/j.rser.2015.11.026
Herrero M, Ibañez E (2018) Green extraction processes, biorefineries and sustainability: recovery of high added-value products from natural sources. J Supercrit Fluids 134:252–259. https://doi.org/10.1016/j.supflu.2017.12.002
Hess TM, Sumberg J, Biggs T, Georgescu M, Haro-Monteagudo D, Jewitt G, Ozdogan M, Marshall M, Thenkabail P, Daccache A, Marin F, Knox JW (2016) A sweet deal? Sugarcane, water and agricultural transformation in sub-saharan africa. Glob Environ Change 39:181–194. https://doi.org/10.1016/j.gloenvcha.2016.05.003
Satlewal A, Agrawal R, Das P, Bhagia S, Pu Y, Puri SK, Ramakumar S, Ragauskas AJ (2018) Assessing the facile pretreatments of bagasse for efficient enzymatic conversion and their impacts on structural and chemical properties. ACS Sustain Chem Eng 7(1):1095–1104
Pin TC, Nakasu PY, Mattedi S, Rabelo SC, Costa AC (2019) Screening of protic ionic liquids for sugarcane bagasse pretreatment. Fuel 235:1506–1514
Kumar A, Negi YS, Choudhary V, Bhardwaj NK (2014) Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J Mater Phys Chem 2(1):1–8
Wulandari WT, Rochliadi A, Arcana IM (2016) Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf Ser Mater Sci Eng 107(1):012045
Oliveira FBd, Bras J, Pimenta MTB, Curvelo AAdS, Belgacem MN (2016) Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Ind Crops Prod 93:48–57. https://doi.org/10.1016/j.indcrop.2016.04.064
de Miranda CMR, Neta JV, Ferreira LFR, Júnior WAG, do Nascimento CS, Gomes EDB, Mattedi S, Soares CM, Lima ÁS (2019) Pineapple crown delignification using low-cost ionic liquid based on ethanolamine and organic acids. Carbohyd Polym 206:302–308
Pérez-Pimienta JA, Icaza-Herrera JP, Méndoza-Pérez JA, González-Álvarez V, Méndez-Acosta HO, Arreola-Vargas J (2019) Mild reaction conditions induce high sugar yields during the pretreatment of agave tequilana bagasse with 1-ethyl-3-methylimidazolium acetate. Biores Technol 275:78–85
Daems N, Sheng X, Vankelecom IF, Pescarmona PP (2014) Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J Mater Chem A 2(12):4085–4110
Sun F, Wang L, Peng Y, Gao J, Pi X, Qu Z, Zhao G, Qin Y (2018) Converting biomass waste into microporous carbon with simultaneously high surface area and carbon purity as advanced electrochemical energy storage materials. Appl Surf Sci 436:486–494. https://doi.org/10.1016/j.apsusc.2017.12.067
Guldi DM, Sgobba V (2011) Carbon nanostructures for solar energy conversion schemes. Chem Commun (Camb) 47(2):606–610. https://doi.org/10.1039/c0cc02411b
Peter KT, Vargo JD, Rupasinghe TP, De Jesus A, Tivanski AV, Sander EA, Myung NV, Cwiertny DM (2016) Synthesis, optimization, and performance demonstration of electrospun carbon nanofiber–carbon nanotube composite sorbents for point-of-use water treatment. ACS Appl Mater Interfaces 8(18):11431–11440. https://doi.org/10.1021/acsami.6b01253
Dresselhaus MS, Dresselhaus G, Eklund PC (1996) Science of fullerenes and carbon nanotubes: their properties and applications. Academic Press, Cambridge
Misnon II, Zain NKM, Jose R (2018) Conversion of oil palm kernel shell biomass to activated carbon for supercapacitor electrode application. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0196-y
Ritter U, Tsierkezos NG, Prylutskyy YI, Matzui LY, Gubanov VO, Bilyi MM, Davydenko MO (2012) Structure–electrical resistivity relationship of n-doped multi-walled carbon nanotubes. J Mater Sci 47(5):2390–2395. https://doi.org/10.1007/s10853-011-6059-6
Jana D, Sun C-L, Chen L-C, Chen K-H (2013) Effect of chemical doping of boron and nitrogen on the electronic, optical, and electrochemical properties of carbon nanotubes. Prog Mater Sci 58(5):565–635
Ombaka LM, Ndungu PG, Nyamori VO (2015) Tuning the nitrogen content and surface properties of nitrogen-doped carbon nanotubes synthesized using a nitrogen-containing ferrocenyl derivative and ethylbenzoate. J Mater Sci 50(3):1187–1200. https://doi.org/10.1007/s10853-014-8675-4
Lin J, Shang Y, Lin X, Yang L, Yu A (2016) Study on nitrogen-doped carbon nanotubes for vanadium redox flow battery application. Int J Electrochem Sci 11(1):665–674
Wu M-S, Ceng Z-Z (2016) Bamboo-like nitrogen-doped carbon nanotubes formed by direct pyrolysis of prussian blue analogue as a counter electrode material for dye-sensitized solar cells. Electrochim Acta 191:895–901. https://doi.org/10.1016/j.electacta.2016.01.123
Martin-Martinez M, Ribeiro RS, Machado BF, Serp P, Morales-Torres S, Silva AMT, Figueiredo JL, Faria JL, Gomes HT (2016) Role of nitrogen doping on the performance of carbon nanotube catalysts: a catalytic wet peroxide oxidation application. ChemCatChem 8(12):2068–2078. https://doi.org/10.1002/cctc.201600123
Zhang L-M, Sui X-L, Zhao L, Zhang J-J, Gu D-M, Wang Z-B (2016) Nitrogen-doped carbon nanotubes for high-performance platinum-based catalysts in methanol oxidation reaction. Carbon 108:561–567. https://doi.org/10.1016/j.carbon.2016.07.059
Kumar M, Ando Y (2010) Chemical vapor deposition of carbon nanotubes: a review on growth mechanism and mass production. J Nanosci Nanotechnol 10(6):3739–3758. https://doi.org/10.1166/jnn.2010.2939
Hussain S, Amade R, Moreno H, Bertran E (2014) RF-PECVD growth and nitrogen plasma functionalization of CNTs on copper foil for electrochemical applications. Diam Relat Mater 49:55–61. https://doi.org/10.1016/j.diamond.2014.08.006
Keru G, Ndungu PG, Nyamori VO (2013) Nitrogen-doped carbon nanotubes synthesised by pyrolysis of (4-{[(pyridine-4-yl)methylidene]amino}phenyl)ferrocene. J Nanomater 2013:1–7. https://doi.org/10.1155/2013/750318
Zaytseva O, Neumann G (2016) Carbon nanomaterials: production, impact on plant development, agricultural and environmental applications. Chem Biol Technol Agric. https://doi.org/10.1186/s40538-016-0070-8
Chu S, Majumdar A (2012) Opportunities and challenges for a sustainable energy future. Nature 488(7411):294–303
Fritsch C, Staebler A, Happel A, Cubero Márquez M, Aguiló-Aguayo I, Abadias M, Gallur M, Cigognini I, Montanari A, López M (2017) Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: a review. Sustainability 9(8):1492
Goodell B, Xie X, Qian Y, Daniel G, Peterson M, Jellison J (2008) Carbon nanotubes produced from natural cellulosic materials. J Nanosci Nanotechnol 8(5):2472–2474
Kang Z, Wang E, Mao B, Su Z, Chen L, Xu L (2005) Obtaining carbon nanotubes from grass. Nanotechnology 16(8):1192–1195
Liu H, Ren M, Qu J, Feng Y, Song X, Zhang Q, Cong Q, Yuan X (2017) A cost-effective method for recycling carbon and metals in plants: synthesizing nanomaterials. Environ Sci Nano 4(2):461–469
Bernd MGS, Bragança SR, Heck N, Filho LC (2017) Synthesis of carbon nanostructures by the pyrolysis of wood sawdust in a tubular reactor. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2016.11.003
Alves JO, Tenório JAS, Zhuo C, Levendis YA (2012) Characterization of nanomaterials produced from sugarcane bagasse. J Mater Res Technol 1(1):31–34
Wei L, Sevilla M, Fuertes AB, Mokaya R, Yushin G (2011) Hydrothermal carbonization of abundant renewable natural organic chemicals for high-performance supercapacitor electrodes. Adv Energy Mater 1(3):356–361. https://doi.org/10.1002/aenm.201100019
Zhu C, Akiyama T (2016) Cotton derived porous carbon via an mgo template method for high performance lithium ion battery anodes. Green Chem 18(7):2106–2114
De S, Balu AM, Waal JC, van Luque R (2015) Biomass-derived porous carbon materials: synthesis and catalytic applications. ChemCatChem 7(11):1608–1629. https://doi.org/10.1002/cctc.201500081
Hofsetz K, Silva MA (2012) Brazilian sugarcane bagasse: energy and non-energy consumption. Biomass Bioenerg 46:564–573
Melati RB, Shimizu FL, Oliveira G, Pagnocca FC, de Souza W, Sant’Anna C, Brienzo M (2018) Key factors affecting the recalcitrance and conversion process of biomass. BioEnergy Res. https://doi.org/10.1007/s12155-018-9941-0
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109(12):6712–6728. https://doi.org/10.1021/cr9001947
Equihua-Sánchez M, Barahona-Pérez LF (2019) Physical and chemical characterization of agave tequilana bagasse pretreated with the ionic liquid 1-ethyl-3-methylimidazolium acetate. Waste Biomass Valoriz 10(5):1285–1294
Wang H, Gurau G, Rogers RD (2012) Ionic liquid processing of cellulose. Chem Soc Rev 41(4):1519–1537. https://doi.org/10.1039/c2cs15311d
Chiappe C, Pieraccini D (2005) Ionic liquids: solvent properties and organic reactivity. J Phys Org Chem 18(4):275–297
Ozokwelu D, Zhang S, Okafor O, Cheng W, Litombe N (2017) Novel catalytic and separation processes based on ionic liquids. Elsevier, Amsterdam
Morales-delaRosa S, Campos-Martin JM, Fierro JLG (2012) High glucose yields from the hydrolysis of cellulose dissolved in ionic liquids. Chem Eng J 181–182:538–541. https://doi.org/10.1016/j.cej.2011.11.061
Mugadza K, Nyamori VO, Mola GT, Simoyi RH, Ndungu PG (2017) Low temperature synthesis of multiwalled carbon nanotubes and incorporation into an organic solar cell. J Exp Nanosci 12(1):363–383. https://doi.org/10.1080/17458080.2017.1357842
Ombaka LM, Ndungu PG, Nyamori VO (2014) Tuning the nitrogen content and surface properties of nitrogen-doped carbon nanotubes synthesized using a nitrogen-containing ferrocenyl derivative and ethylbenzoate. J Mater Sci 50(3):1187–1200. https://doi.org/10.1007/s10853-014-8675-4
Conroy D, Moisala A, Cardoso S, Windle A, Davidson J (2010) Carbon nanotube reactor: ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up. Chem Eng Sci 65(10):2965–2977. https://doi.org/10.1016/j.ces.2010.01.019
Castro C, Pinault M, Porterat D, Reynaud C, Mayne-L’Hermite M (2013) The role of hydrogen in the aerosol-assisted chemical vapor deposition process in producing thin and densely packed vertically aligned carbon nanotubes. Carbon 61:585–594. https://doi.org/10.1016/j.carbon.2013.05.040
Xu Y, Ma Y, Liu Y, Feng S, He D, Haghi-Ashtiani P, Dichiara A, Zimmer L, Bai J (2018) Evolution of nanoparticles in the gas phase during the floating chemical vapor deposition synthesis of carbon nanotubes. J Phys Chem C 122(11):6437–6446. https://doi.org/10.1021/acs.jpcc.8b00451
Aqel A, Abou El-Nour KMM, Ammar RAA, Al-Warthan A (2012) Carbon nanotubes, science and technology part (i) structure, synthesis and characterization. Arab J Chem 5(1):1–23. https://doi.org/10.1016/j.arabjc.2010.08.022
Mugadza K, Ndungu PG, Stark A, Nyamori VO (2019) Ionic liquids and cellulose: innovative feedstock for synthesis of carbon nanostructured material. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2019.06.012
Mori S, Suzuki M (2008) Effect of oxygen and hydrogen addition on the low-temperature synthesis of carbon nanofibers using a low-temperature co/ar dc plasma. Diam Relat Mater 17(6):999–1002. https://doi.org/10.1016/j.diamond.2008.02.035
Belin T, Epron F (2005) Characterization methods of carbon nanotubes: a review. Mater Sci Eng B 119(2):105–118. https://doi.org/10.1016/j.mseb.2005.02.046
Lee CJ, Lyu SC, Kim H-W, Lee JH, Cho KI (2002) Synthesis of bamboo-shaped carbon–nitrogen nanotubes using c2h2–nh3–fe(co)5 system. Chem Phys Lett 359(1–2):115–120. https://doi.org/10.1016/S0009-2614(02)00655-3
Hou P-X, Liu C, Cheng H-M (2008) Purification of carbon nanotubes. Carbon 46(15):2003–2025
Hsieh Y-C, Chou Y-C, Lin C-P, Hsieh T-F, Shu C-M (2010) Thermal analysis of multi-walled carbon nanotubes by kissinger’s corrected kinetic equation. Aerosol Air Qual Res 10(3):212–218
Mahajan A, Kingon A, Kukovecz Á, Konya Z, Vilarinho PM (2013) Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mater Lett 90:165–168. https://doi.org/10.1016/j.matlet.2012.08.120
Nxumalo EN, Chabalala VP, Nyamori VO, Witcomb MJ, Coville NJ (2010) Influence of methylimidazole isomers on ferrocene-catalysed nitrogen doped carbon nanotube synthesis. J Organomet Chem 695(10–11):1451–1457
Bom D, Andrews R, Jacques D, Anthony J, Chen B, Meier MS, Selegue JP (2002) Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett 2(6):615–619
Santangelo S, Lanza M, Milone C (2013) Evaluation of the overall crystalline quality of amorphous carbon containing multiwalled nanotubes. The Journal of Physical Chemistry C 117(9):4815–4823. https://doi.org/10.1021/jp310014w
Jorio A, Pimenta MA, Souza Filho AG, Saito R, Dresselhaus G, Dresselhaus MS (2003) Characterizing carbon nanotube samples with resonance raman scattering. New J Phys 5:1–17. https://doi.org/10.1088/1367-2630/5/1/139
Costa S, Borowiak-Palen E, Kruszynska M, Bachmatiuk A, Kalenczuk RJ (2008) Characterization of carbon nanotubes by raman spectroscopy. Mater Sci-Poland 26(2):433–441
Ferrari AC, Robertson J (2001) Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys Rev B. https://doi.org/10.1103/physrevb.64.075414
Liu Y, Pan C, Wang J (2004) Raman spectra of carbon nanotubes and nanofibers prepared by ethanol flames. J Mater Sci 39(3):1091–1094. https://doi.org/10.1023/b:jmsc.0000012952.20840.09
Wepasnick KA, Smith BA, Bitter JL, Howard Fairbrother D (2010) Chemical and structural characterization of carbon nanotube surfaces. Anal Bioanal Chem 396(3):1003–1014. https://doi.org/10.1007/s00216-009-3332-5
Osswald S, Havel M, Gogotsi Y (2007) Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J Raman Spectrosc 38(6):728–736
Hernadi K, Fonseca A, Nagy JB, Siska A, Kiricsi I (2000) Production of nanotubes by the catalytic decomposition of different carbon-containing compounds. Appl Catal A 199(2):245–255
Cao A, Xu C, Liang J, Wu D, Wei B (2001) X-ray diffraction characterization on the alignment degree of carbon nanotubes. Chem Phys Lett 344(1):13–17. https://doi.org/10.1016/S0009-2614(01)00671-6
Lambin P, Loiseau A, Culot C, Biro L (2002) Structure of carbon nanotubes probed by local and global probes. Carbon 40(10):1635–1648
Reznik D, Olk CH, Neumann DA, Copley JRD (1995) X-ray powder diffraction from carbon nanotubes and nanoparticles. Phys Rev B Condens Matter 52(1):116–124. https://doi.org/10.1103/physrevb.52.116
Khani H, Moradi O (2013) Influence of surface oxidation on the morphological and crystallographic structure of multi-walled carbon nanotubes via different oxidants. J Nanostruct Chem 3(1):73–81
Titus E, Ali N, Cabral G, Gracio J, Ramesh Babu P, Jackson MJ (2006) Chemically functionalized carbon nanotubes and their characterization using thermogravimetric analysis, fourier transform infrared, and raman spectroscopy. J Mater Eng Perform 15(2):182–186. https://doi.org/10.1361/105994906x95841
Steven E, Saleh WR, Lebedev V, Acquah SF, Laukhin V, Alamo RG, Brooks JS (2013) Carbon nanotubes on a spider silk scaffold. Nat Commun 4:2435
Mombeshora ET, Ndungu PG, Jarvis AL, Nyamori VO (2017) Oxygen-modified multiwalled carbon nanotubes: physicochemical properties and capacitor functionality. Int J Energy Res 41(8):1182–1201
He C, Li L, Zhang T, Sun F, Wang C, Lin Y (2016) Nitrogen-doped amorphous carbon with effective electrocatalytic activity toward oxygen reduction reaction. Mater Res Bull 84:118–123. https://doi.org/10.1016/j.materresbull.2016.07.025
Wang Y-G, Li H-Q, Xia Y-Y (2006) Ordered whiskerlike polyaniline grown on the surface of mesoporous carbon and its electrochemical capacitance performance. Adv Mater 18(19):2619–2623. https://doi.org/10.1002/adma.200600445
Wickramaratne NP, Xu J, Wang M, Zhu L, Dai L, Jaroniec M (2014) Nitrogen enriched porous carbon spheres: attractive materials for supercapacitor electrodes and co2 adsorption. Chem Mater 26(9):2820–2828. https://doi.org/10.1021/cm5001895
Acknowledgements
The authors wish to thank the Sugar Milling Research Institute (SMRI) and the National Research Foundation-The World Academy of Science (NRF-TWAS) for the supply of the feedstock and funding, respectively, and Dr. ET Mombeshora for proofreading the manuscript. This work is based on research supported in part (Grant Number: 116610, to KM, Grant Number: 103979, to VON and Grant Number: 115465, to AS) by the National Research Foundation of South Africa. Also the Eskom Tertiary Education Support Programme (TESP) funded this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mugadza, K., Ndungu, P.G., Stark, A. et al. Conversion of residue biomass into value added carbon materials: utilisation of sugarcane bagasse and ionic liquids. J Mater Sci 54, 12476–12487 (2019). https://doi.org/10.1007/s10853-019-03800-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03800-5