Abstract
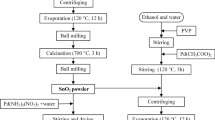
Oxygen adsorption on the surface of particles plays a key role in gas sensing for SnO2-based resistive-type gas sensors in a humid atmosphere. In this study, we added an extremely small amount of ZrO2 on the SnO2 surface to activate oxygen adsorption and enhance the sensor response of a SnO2-based gas sensor in a humid atmosphere. We evaluated the oxygen adsorption properties and sensor response to 200 ppm H2 in a humid atmosphere (96%) based on variations in the measured electrical resistance. The adsorption of O− and O2− ions was activated by a small loading (0.033 mol%) of ZrO2, and the resulting ZrO2–SnO2 composite nanoparticles were in the deep electron-depleted state. This led to a high sensor response of ZrO2–SnO2 to H2 in a humid atmosphere. The results demonstrate that surface modification using an extremely small amount of ZrO2 was effective in improving the response of a SnO2-based gas sensor in a humid atmosphere.






Similar content being viewed by others
References
Homepage of Figaro Engineering Inc. http://www.figaro.co.jp/en/. Accessed 22 Aug 2018
Homepage of Nissha FIS, Inc. http://www.fisinc.co.jp/en/. Accessed 22 Aug 2018
Yamazoe N, Fuchigami J, Kishikawa M, Seiyama T (1979) Interactions of tin oxide surface with O2, H2O and H2. Surf Sci 86:335–344. https://doi.org/10.1016/0039-6028(79)90411-4
Barsan N, Weimar U (2001) Conduction model of metal oxide gas sensors. J Electroceram 7:143–167. https://doi.org/10.1023/A:1014405811371
Koziej D, Bârsan N, Weimar U, Szuber J, Shimanoe K, Yamazoe N (2005) Water–oxygen interplay on tin dioxide surface: implication on gas sensing. Chem Phys Lett 410:321–323. https://doi.org/10.1016/j.cplett.2005.05.107
Yue J, Jiang X, Yu A (2013) Adsorption of the OH group on SnO2 (110) oxygen bridges: a molecular dynamics and density functional theory study. J Phys Chem C 117:9962–9969. https://doi.org/10.1021/jp4022294
Santarossa G, Hahn K, Baiker A (2013) Free energy and electronic properties of water adsorption on the SnO2 (110) Surface. Langmuir 29:5487–5499. https://doi.org/10.1021/la400313a
Wicker S, Guiltat M, Weimar U, Hémeryck A, Barsan N (2017) Ambient humidity influence on CO detection with SnO2 gas sensing materials. A combined DRIFTS/DFT investigation. J Phys Chem C 121:25064–25073. https://doi.org/10.1021/acs.jpcc.7b06253
Zhu H, Li Q, Ren Y, Gao Q, Chen J, Wang N, Deng J, Xing X (2018) A new insight into cross-sensitivity to humidity of SnO2 sensor. Small 14:1703974. https://doi.org/10.1002/smll.201703974
Suematsu K, Ma N, Watanabe K, Yuasa M, Kida T, Shimanoe K (2018) Effect of humid aging on the oxygen adsorption in SnO2 gas sensors. Sensors 18:1–11. https://doi.org/10.3390/s18010254
Kim HR, Haensch A, Kim ID, Barsan N, Weimar U, Lee JH (2011) The role of NiO doping in reducing the impact of humidity on the performance of SnO2-based gas sensors: synthesis strategies, and phenomenological and spectroscopic studies. Adv Funct Mater 21:4456–4463. https://doi.org/10.1002/adfm.201101154
Shin J, Choi SJ, Lee I, Youn DY, Park CO, Lee JH, Tuller HL, Kim ID (2013) Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv Funct Mater 23:2357–2367. https://doi.org/10.1002/adfm.201202729
Suematsu K, Ma N, Yuasa M, Kida T, Shimanoe K (2015) Surface-modification of SnO2 nanoparticles by incorporation of Al for the detection of combustible gases in a humid atmosphere. RSC Adv 5:86347–86354. https://doi.org/10.1039/c5ra17556a
Suematsu K, Sasaki M, Ma N, Yuasa M, Shimanoe K (2016) Antimony-doped tin dioxide gas sensors exhibiting high stability in the sensitivity to humidity changes. ACS Sens 1:913–920. https://doi.org/10.1021/acssensors.6b00323
Zito CA, Perfecto TM, Volanti DP (2017) Palladium-loaded hierarchical flower-like tin dioxide structure as chemosensor exhibiting high ethanol response in humid conditions. Adv Mater Interfaces 4:1–11. https://doi.org/10.1002/admi.201700847
Kwak CH, Kim TH, Jeong SY, Yoon JW, Kim JS, Lee JH (2018) Humidity-independent oxide semiconductor chemiresistors using terbium-doped SnO2 Yolk–Shell spheres for real-time breath analysis. ACS Appl Mater Interfaces 10:18886–18894. https://doi.org/10.1021/acsami.8b04245
Rogers PH, Benkstein KD, Semancik S (2012) Machine learning applied to chemical analysis: sensing multiple biomarkers in simulated breath using a temperature-pulsed electronic-nose. Anal Chem 84:9774–9781. https://doi.org/10.1021/ac301687j
Choi SJ, Jang BH, Lee SJ, Min BK, Rothschild A, Kim ID (2014) Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl Mater Interfaces 6:2588–2597. https://doi.org/10.1021/am405088q
Güntner AT, Koren V, Chikkadi K, Righettoni M, Pratsinis SE (2016) E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens 1:528–535. https://doi.org/10.1021/acssensors.6b00008
Zito CA, Perfecto TM, Volanti DP (2017) Impact of reduced graphene oxide on the ethanol sensing performance of hollow SnO2 nanoparticles under humid atmosphere. Sens Actuators B 244:466–474. https://doi.org/10.1016/j.snb.2017.01.015
Korotchenkov G, Brynzari V, Dmitriev S (1999) Electrical behavior of SnO2 thin films in humid atmosphere. Sens Actuators B 54:197–201. https://doi.org/10.1016/S0925-4005(99)00016-7
Großmann K, Wicker S, Weimar U, Barsan N (2013) Impact of Pt additives on the surface reactions between SnO2, water vapour, CO and H2; An operando investigation. Phys Chem Chem Phys 15:19151–19158. https://doi.org/10.1039/c3cp52782d
Ma N, Suematsu K, Yuasa M, Kida T, Shimanoe K (2015) Effect of water vapor on Pd-loaded SnO2 nanoparticles gas sensor. ACS Appl Mater Interfaces 7:5863–5869. https://doi.org/10.1021/am509082w
Ma N, Suematsu K, Yuasa M, Shimanoe K (2015) Pd size effect on the gas sensing properties of Pd-loaded SnO2 in humid atmosphere. ACS Appl Mater Interfaces 7:15618–15625. https://doi.org/10.1021/acsami.5b04380
Choi KI, Kim HJ, Kang YC, Lee JH (2014) Ultraselective and ultrasensitive detection of H2S in highly humid atmosphere using CuO-loaded SnO2 hollow spheres for real-time diagnosis of halitosis. Sens Actuators B 194:371–376. https://doi.org/10.1016/j.snb.2013.12.111
Yoon JW, Kim JS, Kim TH, Hong YJ, Kang YC, Lee JH (2016) A new strategy for humidity independent oxide chemiresistors: dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small 12:4229–4240. https://doi.org/10.1002/smll.201601507
Yamazoe N, Shimanoe K (2008) Roles of shape and size of component crystals in semiconductor gas sensors. J Electrochem Soc 155:J93–J98. https://doi.org/10.1149/1.2832662
Yamazoe N, Suematsu K, Shimanoe K (2012) Extension of receptor function theory to include two types of adsorbed oxygen for oxide semiconductor gas sensors. Sens Actuators B 163:128–135. https://doi.org/10.1016/j.snb.2012.01.020
Suematsu K, Yuasa M, Kida T, Yamazoe N, Shimanoe K (2014) Determination of oxygen adsorption species on SnO2: exact analysis of gas sensing properties using a sample gas pretreatment system. J Electrochem Soc 161:B123–B128. https://doi.org/10.1149/2.004406jes
Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI), Grant Numbers JP16H04219 and JP17K17941. We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Suematsu, K., Uchino, H., Mizukami, T. et al. Oxygen adsorption on ZrO2-loaded SnO2 gas sensors in humid atmosphere. J Mater Sci 54, 3135–3143 (2019). https://doi.org/10.1007/s10853-018-3020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-3020-y