Abstract
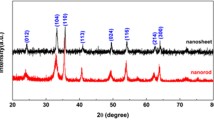
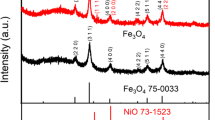
Fe3O4/NiFe2O4 nanosheets as anode materials for lithium-ion batteries have been successfully prepared by a facile one-step dealloying method of Al–Fe–Ni alloy precursor. Herein, the XRD results and XPS spectra indicated that the product consists of cubic NiFe2O4 and Fe3O4. Scanning electronic microscopy and transmission electron microscopy images showed the homogenous distribution of Fe3O4/NiFe2O4 in the quasi-hexagonal nanosheets rather than irregular clusters. Besides nanostructures, their electrochemical performances were also enhanced due to the doping of nickel when comparing with the Fe3O4 obtained by dealloying Al–Fe precursor. Cycling and rate tests elicited the DAFN has the Fe3O4/NiFe2O4 product exhibit a higher initial capacity of ~ 1437 mAh g−1 at 200 mA g−1 and modified rate performances, especially they hold a doubled reversible capacity over 500 mAh g−1 after 750 times cycling at 200 mA g−1. Cyclic voltammetry, charge–discharge tests and electrochemical impedance spectra measurements further demonstrated the reinforced initial capacity and cycling stability of the DAFN could be associated with the shortened diffusion pathways and synergistic effect of Fe3O4 and NiFe2O4. The facile and low-cost processing method accompanied with the well-performed product exhibit a promising prospect in the application of lithium-ion storage.





Similar content being viewed by others
References
Goodenough JB, Park KS (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135:1167–1176. https://doi.org/10.1021/ja3091438
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367. https://doi.org/10.1038/35104644
Kang B, Ceder B (2009) Battery materials for ultrafast charging and discharging. Nature 458:190–193. https://doi.org/10.1038/nature07853
Ji LW, Lin Z, Alcoutlabi M, Zhang XW (2011) Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ Sci 4:2682–2699. https://doi.org/10.1039/c0ee00699h
Balogun MS, Qiu WT, Luo Y, Meng H, Mai WJ, Onasanya A, Olaniyi TK, Tong YX (2016) A review of the development of full cell lithium-ion batteries: the impact of nanostructured anode materials. Nano Res 9:2823–2851. https://doi.org/10.1007/s12274-016-1171-1
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2000) Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407:496–499. https://doi.org/10.1038/35035045
Wu HB, Chen JS, Hng HH, Lou XW (2012) Nanostructured metal oxide-based materials as advanced anodes for lithium ion batteries. Nanoscale 4:2526–2542. https://doi.org/10.1039/c2nr11966h
Courtel FM, Duncan H, Abu-Lebdeh Y, Davidson IJ (2011) High capacity anode materials for Li-ion batteries based on spinel metal oxides AMn2O4 (A = Co, Ni and Zn). J Mater Chem 21:10206–10218. https://doi.org/10.1039/c0jm04465b
Liu H, Wang XL, Xu H et al (2017) Nanostructured CoO/NiO/CoNi with tunable morphology for high performance lithium-ion batteries. Dalton Trans 46:11031–11036. https://doi.org/10.1039/c7dt01904a
Balogun MS, Qiu WT, Luo Y et al (2015) Improving the lithium-storage properties of self-grown nickel oxide: a back-up from TiO2 nanoparticles. ChemElectroChem 2:1243–1248. https://doi.org/10.1002/celc.201500146
Huang XH, Tu JP, Zhang B, Li Y, Yuan YF, Wu HM (2006) Electrochemical properties of NiO–Ni nanocomposite as anode material for lithium ion batteries. J Power Sources 161:541–544. https://doi.org/10.1016/j.jpowsour.2006.03.039
Luo Y, Balogun MS, Qiu WT, Zhao RR, Liu P, Tong YX (2015) Sulfurization of FeOOH nanorods on a carbon cloth and their conversion into Fe2O3/Fe3O4–S core–shell nanorods for lithium storage. Chem Commun 51:13016–13019. https://doi.org/10.1039/c5cc04700e
Hua MQ, Xu L, Cui L et al (2018) Hexamethylenetetramine-assisted hydrothermal synthesis of octahedral nickel ferrite oxide nanocrystallines with excellent supercapacitive performance. J Mater Sci 53:7621–7636. https://doi.org/10.1007/s10853-018-2052-7
Thankachan RM, Rahman MM, Sultana I, Glushenkov AM, Thomas S, Kalarikkal N, Chen Y (2015) Enhanced lithium storage in ZnFe2O4–C nanocomposite produced by a low-energy ball milling. J Power Sources 282:462–470. https://doi.org/10.1016/j.jpowsour.2015.02.039
Zhang H, Zhao YM, Wen MM, Dong YZ, Fan QH, Kuang Q, Liu HT, Lian X (2018) A new sodium ferrous orthophosphate NaxFe4(PO4)3 as anode materials for sodium-ion batteries. J Mater Sci 53:8385–8397. https://doi.org/10.1007/s10853-018-2128-4
Nie LY, Wang HJ, Ma JJ, Liu S, Yuan R (2016) Sulfur-doped ZnFe2O4 nanoparticles with enhanced lithium storage capabilities. J Mater Sci 52:3566–3575. https://doi.org/10.1007/s10853-016-0373-y
Zhai XM, Xu XM, Zhu XL, Zhao YJ, Li JB, Jin HB (2018) Porous layer assembled hierarchical Co3O4 as anode materials for lithium-ion batteries. J Mater Sci 53:1356–1364. https://doi.org/10.1007/s10853-017-1579-3
Takahisa S, Shigeto O, Shin-ichi T, Yun-ichi Y (1996) Study of Li3−xMxN (M: Co, Ni or Cu) system for use as anode material in lithium rechargeable cells. Solid State Ion 86–88:785–789. https://doi.org/10.1016/0167-2738(96)00174-9
Liu DS, Liu DH, Hou BH, Wang YY, Guo JZ, Ning QL, Wu XL (2018) 1D porous MnO@N-doped carbon nanotubes with improved Li-storage properties as advanced anode material for lithium-ion batteries. Electrochim Acta 264:292–300. https://doi.org/10.1016/j.electacta.2018.01.129
Ji XY, Li D, Lu QF, Guo EY, Yao LB, Liu H (2018) Electrospinning preparation of one-dimensional Co2+-doped Li4Ti5O12 nanofibers for high-performance lithium ion battery. Ionics. https://doi.org/10.1007/s11581-018-2453-2
Hao SJ, Zhang BW, Wang Y et al (2018) Hierarchical three-dimensional Fe3O4@porous carbon matrix/graphene anodes for high performance lithium ion batteries. Electrochim Acta 260:965–973. https://doi.org/10.1016/j.electacta.2017.12.078
Wang JX, Xiong Y, Zhang XH (2016) Rational synthesis of NiCo2O4 meso-structures for high-rate supercapacitors. J Mater Sci 52:3678–3686. https://doi.org/10.1007/s10853-016-0658-1
Li J, Xu SJ, Huang S, Lu L, Lan LF, Li SF (2017) In situ synthesis of Fe(1−x)CoxF3/MWCNT nanocomposites with excellent electrochemical performance for lithium-ion batteries. J Mater Sci 53:2697–2708. https://doi.org/10.1007/s10853-017-1685-2
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2001) Searching for new anode materials for the Li-ion technology: time to deviate from the usual path. J Power Sources 97–98:235–239. https://doi.org/10.1016/S0378-7753(01)00508-0
Shin WH, Jeong HM, Kim BG, Kang JK, Choi JW (2012) Nitrogen-doped multiwall carbon nanotubes for lithium storage with extremely high capacity. Nano Lett 12:2283–2288. https://doi.org/10.1021/nl3000908
Feng XS, Huang Y, Chen XF, Wei C, Zhang X, Chen MH (2017) Hierarchical CoFe2O4/NiFe2O4 nanocomposites with enhanced electrochemical capacitive properties. J Mater Sci 53:2648–2657. https://doi.org/10.1007/s10853-017-1735-9
Walle MD, Zhang ZF, Zhang MY, You XL, Li YJ, Liu YN (2017) Hierarchical 3D nitrogen and phosphorous codoped graphene/carbon nanotubes–sulfur composite with synergistic effect for high performance of lithium–sulfur batteries. J Mater Sci 53:2685–2696. https://doi.org/10.1007/s10853-017-1678-1
Qiu WT, Balogun MS, Luo Y et al (2016) Three-dimensional Fe3O4 nanotube array on carbon cloth prepared from a facile route for lithium ion batteries. Electrochim Acta 193:32–38. https://doi.org/10.1016/j.electacta.2016.01.173
Yuan CZ, Wu HB, Xie Y, Lou XW (2014) Mixed transition-metal oxides: design, synthesis, and energy-related applications. Angew Chem Int Ed 53:1488–1504. https://doi.org/10.1002/anie.201303971
Li XF, Dhanabalan A, Wang CL (2011) Enhanced electrochemical performance of porous NiO–Ni nanocomposite anode for lithium ion batteries. J Power Sources 196:9625–9630. https://doi.org/10.1016/j.jpowsour.2011.06.097
Wu SH, Fu GL, Lv WQ et al (2018) A single-step hydrothermal route to 3D hierarchical Cu2O/CuO/rGO nanosheets as high-performance anode of lithium-ion batteries. Small 14:1702667. https://doi.org/10.1002/smll.201702667
Su DW, Kim HS, Kim WS, Wang GX (2012) Mesoporous nickel oxide nanowires: hydrothermal synthesis, characterisation and applications for lithium-ion batteries and supercapacitors with superior performance. Chem Eur J 18:8224–8229. https://doi.org/10.1002/chem.201200086
Qin J, Liu DY, Zhao NQ et al (2018) Fabrication of Sn-core/CNT-shell nanocable anchored interconnected carbon networks as anode material for lithium ion batteries. Mater Lett 212:94–97. https://doi.org/10.1016/j.matlet.2017.10.011
Han JZ, Qin J, Guo LC et al (2018) Ultrasmall Fe2GeO4 nanodots anchored on interconnected carbon nanosheets as high-performance anode materials for lithium and sodium ion batteries. Appl Surf Sci 427:670–679. https://doi.org/10.1016/j.apsusc.2017.08.026
Helle S, Pedron M, Assouli B, Davis B, Guay D, Roué L (2010) Structure and high-temperature oxidation behaviour of Cu–Ni–Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminium electrolysis. Corros Sci 52:3348–3355. https://doi.org/10.1016/j.corsci.2010.06.011
Xu CX, Wang RY, Zhang Y, Ding Y (2010) A general corrosion route to nanostructured metal oxides. Nanoscale 2:906–909. https://doi.org/10.1039/b9nr00351g
Liu H, Wang XL, Wang JX, Xu H, Yu WS, Dong XT, Zhang HB, Wang LM (2017) Hierarchical porous CoNi/CoO/NiO composites derived from dealloyed quasicrystals as advanced anodes for lithium-ion batteries. Scripta Mater 139:30–33. https://doi.org/10.1016/j.scriptamat.2017.06.011
Liu H, Wang XL, Wang JX, Xu H, Yu WS, Dong XT, Zhang HB, Wang LM (2017) High electrochemical performance of nanoporous Fe3O4/CuO/Cu composites synthesized by dealloying Al–Cu–Fe quasicrystal. J Alloys Compd 729:360–369. https://doi.org/10.1016/j.jallcom.2017.09.111
Zhang J, Ling YH, Gao WB, Wang S, Li JT (2013) Enhanced photoelectrochemical water splitting on novel nanoflake WO3 electrodes by dealloying of amorphous Fe–W alloys. J Mater Chem A 1:10677–10685. https://doi.org/10.1039/c3ta12273e
Huber GW, Shabaker JW, Dumesic JA (2003) Raney Ni-Sn catalyst for H2 production from biomass-derived hydrocarbons. Science 300:2075–2077. https://doi.org/10.1126/science.1085597
Kittler S, Greulich C, Diendorf J, Köller M, Epple M (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22:4548–4554. https://doi.org/10.1021/cm100023p
Biesinger MC, Payne BP, Grosvenor AP, Lau LWM, Gerson AR, Smart RSC (2011) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci 257:2717–2730. https://doi.org/10.1016/j.apsusc.2010.10.051
Anderson JF, Kuhn M, Diebold U (1996) Epitaxially grown Fe3O4 thin films: an XPS study. Surf Sci Spectra 4:266–272. https://doi.org/10.1116/1.1247796
Liu PB, Huang Y, Zhang X (2014) Enhanced electromagnetic absorption properties of reduced graphene oxide-polypyrrole with NiFe2O4 particles prepared with simple hydrothermal method. Mater Lett 120:123–146. https://doi.org/10.1016/j.matlet.2014.01.054
Chen LY, Dai H, Shen YM, Bai JF (2010) Size-controlled synthesis and magnetic properties of NiFe2O4 hollow nanospheres via a gel-assistant hydrothermal route. J Alloys Compd 491:L33–L38. https://doi.org/10.1016/j.jallcom.2009.11.031
Cheng FY, Su CH, Yang YS, Yeh CS, Tsai CY, Wu CL, Wu MT, Shieh DB (2005) Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 26:729–738. https://doi.org/10.1016/j.biomaterials.2001.03.016
Langell MA, Wilson D (2014) XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl Surf Sci 303:6–13. https://doi.org/10.1016/j.apsusc.2014.02.006
Ren A, Liu CB, Hong YZ, Shi WD, Lin S, Li P (2014) Enhanced visible-light-driven photocatalytic activity for antibiotic degradation using magnetic NiFe2O4/Bi2O3 heterostructures. Chem Eng J 258:301–308. https://doi.org/10.1016/j.cej.2014.07.071
Hao Q, Li MH, Jia SZ, Zhao XY, Xu CX (2013) Controllable preparation of Co3O4 nanosheets and their electrochemical performance for Li-ion batteries. RSC Adv 3:7850–7854. https://doi.org/10.1039/c3ra23448g
Long X, Wang ZL, Xiao S, An YM, Yang SH (2016) Transition metal based layered double hydroxides tailored for energy conversion and storage. Mater Today 19:213–226. https://doi.org/10.1016/j.mattod.2015.10.006
Cherian CT, Sundaramurthy J, Reddy MV et al (2013) Morphologically robust NiFe2O4 nanofibers as high capacity Li-ion battery anode material. ACS Appl Mater Interfaces 5:9957–9963. https://doi.org/10.1021/am401779p
Zhou GM, Wang DW, Li F et al (2010) Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem Mater 22:5306–5313. https://doi.org/10.1021/cm101532x
Zhao XY, Xia DG, Zheng K (2012) Fe3O4/Fe/Carbon composite and its application as anode material for lithium-ion batteries. ACS Appl Mater Interfaces 4:1350–1356. https://doi.org/10.1021/am201617j
Li H, Huang XJ, Chen LQ (1999) Direct imaging of the passivating film and microstructure of nanometer-scale SnO anodes in lithium rechargeable batteries. Electrochem Solid-State Lett 1:241–243. https://doi.org/10.1149/1.1390699
Li H, Shi LH, Lu W, Huang XJ, Chen LQ (2001) Studies on capacity loss and capacity fading of nanosized SnSb alloy anode for Li-ion batteries. J Electrochem Soc 148:A915–A922. https://doi.org/10.1149/1.1383070
Stjerndahl M, Bryngelsson H, Gustafsson T, Vaughey JT, Thackeray MM, Edström K (2007) Surface chemistry of intermetallic AlSb-anodes for Li-ion batteries. Electrochim Acta 52:4947–4955. https://doi.org/10.1016/j.electacta.2007.01.064
Zhang H (2011) A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J Power Sources 196:13–24. https://doi.org/10.1016/j.jpowsour.2010.07.020
Laruelle S, Grugeon S, Poizot P, Dollé M, Dupont L, Tarascon JM (2002) On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential. J Electrochem Soc 149:A627–A634. https://doi.org/10.1149/1.1467947
Do JS, Weng CH (2005) Preparation and characterization of CoO used as anodic material of lithium battery. J Power Sources 146:482–486. https://doi.org/10.1016/j.jpowsour.2005.03.095
Wang Y, Huang ZG, Wang YY (2015) A new approach to synthesize MoO2@C for high-rate lithium ion batteries. J Mater Chem A 3:21314–21320. https://doi.org/10.1039/c5ta05345e
Kang WP, Tang YB, Li WY, Yang X, Xue HT, Yang QD, Lee CS (2015) High interfacial storage capability of porous NiMn2O4/C hierarchical tremella-like nanostructures as lithium ion battery anode. Nanoscale 7:225–231. https://doi.org/10.1039/c4nr04031g
Zhang LH, Zhu SQ, Cao H, Hou LR, Yuan CZ (2015) Hierarchical porous ZnMn2O4 hollow nanotubes with enhanced lithium storage toward lithium-ion batteries. Chem Eur J 21:10771–10777. https://doi.org/10.1002/chem.201501421
Xi LJ, Wang HE, Lu ZG, Yang SL, Ma RG, Deng JQ, Chung CY (2012) Facile synthesis of porous LiMn2O4 spheres as positive electrode for high-power lithium ion batteries. J Power Sources 198:251–257. https://doi.org/10.1016/j.jpowsour.2011.09.100
Acknowledgements
We gratefully acknowledge the support of this research by the Science and Technology Research Project of the Education Department of Jilin Province during the 13th five-year-plan period (No. 2016-359), the Natural Science Foundation of Jilin Province (No. 20170101128JC), Industrial Technology Research and Development Project of Jilin Province Development and Reform Commission (No. 2017C052-4) and National Natural Science Foundation of China (No. 201601018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, H., Wang, X., Liu, H. et al. Facile synthesis of Fe3O4/NiFe2O4 nanosheets with enhanced Lithium-ion storage by one-step chemical dealloying. J Mater Sci 53, 15631–15642 (2018). https://doi.org/10.1007/s10853-018-2729-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2729-y