Abstract
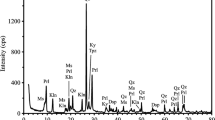
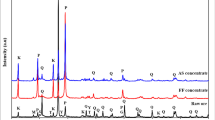
A pyrophyllite [Al2Si4O10(OH)2] ore as an alternative source for alumina (Al2O3) was intensively milled for mechanical activation to increase aluminum extraction by acid leaching method. For this purpose, unmilled and milled ore samples were compared to reveal the changes caused by intensive milling. The samples were also leached in HCl solutions to determine whether mechanical activation increased the alumina extraction or not. The milling considerably disrupted XRD peak intensities of the clay minerals found in the ore, except quartz. After milling just for 30 min, peaks of the minerals were not distinguished, suggesting that all are amorphous. Dehydroxylation of the minerals in the unmilled ore was realized to occur at lower temperature ranges and energy consumed for dehydroxylation significantly reduced with prolonged milling. While aluminum recovery by leaching of the unmilled ore was about 12%, it increased to 73.09% as a result of milling the ore for 50 min. It was concluded that as the milling time is extended, energy needed to undergo dehydroxylation due to amorphization continues to decrease and aluminum recovery reaches higher values, suggesting that the clay minerals in the ore can be converted to mechanically activated state.









Similar content being viewed by others
References
Flint EP, Clarke WF, Newman ES, Shartsis L, Bishop DL, Wells LS (1946) Extraction of alumina from clays and high silica bauxites. J Res Natl Bur Stand 36:63–106
Al-Ajeel AWA, Al-Sindy SI (2006) Alumina recovery from Iraqi kaolinitic clay by hydrochloric acid route. Iraqi Bull Geol Min 2(1):67–76
Bazin C, El Ouassiti K, Ouellet V (2007) Sequential leaching for the recovery of alumina from a Canadian clay. Hydrometallurgy 88:1–4
Numluk P, Chaisena A (2012) Sulfuric acid and ammonium sulfate leaching of alumina from Lampang clay. J Chem 9(3):1364–1372
Erdemoğlu M, Birinci M, Uysal T (2018) Alumina Production from clay Minerals: current reviews. J Polytech 21(2):387–396 (In Turkish)
Habashi F (1999) Textbook of Hydrometallurgy, 2nd edn. Metallurgie Extractive Quebec, Quebec
Li G, Zeng J, Luo J, Liu M, Jiang T, Qiu G (2014) Thermal transformation of pyrophyllite and alkali dissolution behavior of silicon. Appl Clay Sci 99:282–288
Birinci M, Uysal T, Erdemoğlu M, Porgalı E, Barry TS (2017) Acidic leaching of thermally activated pyrophyllite ore from Pütürge (Malatya-Turkey) deposit. In: Proceedings of the 17th Balkan mineral processing congress—BMPC 2017, Antalya, Turkey
Erdemoğlu M, Aydoğan S, Gock E (2009) Effects of intensive grinding on the dissolution of celestite in acidic chloride medium. Miner Eng 22:14–24
Baláž P, Achimovičová M (2006) Mechano-chemical leaching in hydrometallurgy of complex sulphides. Hydrometallurgy 84:60–68
Zhang Z, Yan J, Sheng J (2015) Dry grinding effect on pyrophyllite–quartz natural mixture and its influence on the structural alternation of pyrophyllite. Micron 71:1–6
Lundell GEF, Knowles HB (1929) Use of 8-hydroxyquinoline in separations of aluminum. Bur Stand J Res 5:91–96
Pourghahramani P, Forssberg E (2007) Effects of mechanical activation on the reduction behavior of hematite concentrate. Int J Miner Process 82:96–105
Baláž P (2008) Mechanochemistry in nanoscience and minerals engineering. Springer, Berlin
Uysal T, Mutlu HS, Erdemoğlu M (2016) Effects of mechanical activation of colemanite (Ca2B6O11·5H2O) on its thermal transformations. Int J Miner Process 151:51–58
Kristof E, Juhász ZA (1993) The effect of intensive grinding on the crystal structure of dolomite. Powder Technol 75:145–152
Juhász ZA (1998) Aspects of mechanochemical activation in terms of comminution theory. Coll Surf A Physicochem Eng Asp 141:449–462
Baláž P, Turianicova E, Fabian M, Kleiv RA, Briancin J, Obut A (2008) Structural changes in olivine (Mg, Fe) 2SiO4 mechanically activated in high-energy mills. Int J Miner Process 88:1–6
Juhász ZA (1998) Colloid-chemical aspects of mechanical activation. Part Sci Technol 16(2):145–161
Santos SF, França SCA, Ogasawara T (2011) Method for grinding and delaminating muscovite. Min Sci Technol (China) 21:7–10
Mohammadnejad S, Provis JL, van Deventer JSJ (2014) Effects of grinding on the preg-robbing behaviour of pyrophyllite. Hydrometallurgy 146:154–163
Petrov M, Milošević S (1996) Mechanical activation enthalpy of different minerals. In: Kemal M, Arslan V, Akar A, Canbazoğlu M (eds) Changing scopes in mineral processing. Balkema, Rotterdam, pp 3–7
Mitrović A, Zdujić M (2013) Mechanochemical treatment of Serbian kaolin clay to obtain a highly reactive pozzolana. J Serb Chem Soc 78:579–590
Makó E, Frost RL, Kristóf J, Horváth E (2001) The effect of quartz content on the mechanochemical activation of kaolinite. J Colloid Interface Sci 244:259–364
Hamzaoui R, Muslim F, Guessasma S, Bennabi A, Guillin J (2015) Structural and thermal behavior of proclay kaolinite using high energy ball milling process. Powder Technol 271:228–237
Zhang Z, Wang L (1998) X-ray powder diffraction analysis on characteristics of heating phase transformation of pyrophyllite. J Chinese Ceram Soc 26:618–629
Bragg W, Gibbs RE (1925) The structure of α and β quartz. Proc Roy Soc London A 109(751):405–427
Wahl FM, Grim RE, Graf RB (1961) Phase transformations in silica as examined by continuous X-ray diffraction. Amer Miner 46:196–208
Takashima I (1974) The measurement of inversion temperature of quartz by differential scanning calorimeter (DSC) and its applications to a geothermometer and indicator of growth environment. J Jpn Assoc Min Pet Econ Geol 69:75–80
Hansen T (2018) Quartz inversion. https://digitalfire.com/4sight/glossary/glossary_quartz_ inversion.html. Accessed 4 Feb 2018
Schomburg J (1985) Thermal investigation of pyrophyllites. Thermochim Acta 93:521–524
Sánchez-Soto PJ, Pérez-Rodriguez JL (1989) Thermal-analysis of pyrophyllite transformations. Thermochim Acta 138(2):267–276
Sánchez-Soto PJ, Sobrados I, Sanz J, Pérez-Rodríguez JL (1993) 29-Si and 27-Al magic angle spinning nuclear magnetic resonance study of the thermal transformations of pyrophyllite. J Am Ceram Soc 76:3024–3028
Pérez-Rodríguez JL, Maqueda C, Justo A (1985) Pyrophyllite determination in mineral mixtures. Clays Clay Miner 33(6):563–566
Temuujin J, Okada K, Jadanbaa TS, MacKenzie KJD, Amarsanaa J (2003) Effect of grinding on the leaching behavior of pyrophyllite. J Eur Ceram Soc 23:1277–1282
Kittrick JA (1969) Soil minerals in the Al2O3–SiO2–H2O system and a theory of their formation. Clays Clay Miner 17:157–167
Ganor J, Mogollon JL, Lasaga AC (1995) The effect of pH on kaolinite dissolution rates and on activation energy. Geochim Cosmochim Acta 59(6):1037–1052
Cama J, Metz V, Ganor J (2002) The effect of pH and temperature on kaolinite dissolution rate under acidic conditions. Geochim Cosmochim Acta 66(22):3913–3926
Sokolova TA (2013) Decomposition of clay minerals in model experiments and in soils: possible mechanisms, rates and diagnostics (analysis of literature). Eurasian Soil Sci 46(2):182–197
Acknowledgements
The paper is a part of the Ph.D. study of Turan Uysal conducted under The Scientific and Technological Research Council of Turkey (TÜBİTAK) Doctoral Scholarship which was within the scope of the project which the authors wish to thank TÜBİTAK for supporting the study through the Project 214M432. Supports of İnönü University Coordination Unit for Scientific Research Projects through the Project 2015/44G, Çimsa Cement Inc. (Enis Solakoğlu, Tuğhan Delibaş and Melike Sucu) and İçel Mining Inc., (İbrahim Altuntaş and Nihat Akkaya) for providing pyrophyllite ore samples from Pütürge (Malatya, Turkey), and Prof. Dr. Ömer Bozkaya (Pamukkale University, Turkey) for his valuable comments on the mineral paragenesis of the ore are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Erdemoğlu, M., Birinci, M., Uysal, T. et al. Mechanical activation of pyrophyllite ore for aluminum extraction by acidic leaching. J Mater Sci 53, 13801–13812 (2018). https://doi.org/10.1007/s10853-018-2606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2606-8