Abstract
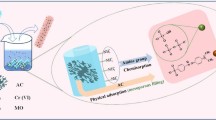
For the treatment of wastewater containing Ag nanoparticles (NPs), PANI/Fe3O4 nanofibers were firstly prepared by a novel self-assemble. And then, the efficiency for the removal of Ag NPs from wastewater was investigated. The magnetic performance of PANI/Fe3O4 nanofibers could be optimized by adjusting the pH of the self-assemblied system. Under pH of 3, the as-prepared nanofibers exhibited the highest magnetism and also displayed good efficiency (> 12 mg g−1) for the removal of Ag NPs. Importantly, the resulted product (PANI/Fe3O4/Ag composite) could act as a catalysis for cleaning durable pollutant, 4-nitrophenol. After 10 cycles, only slight decrease in rate constant was found, indicating excellent reusability. Those approaches provide a new way to merge the recovery of Ag NPs as pollutants and reuse of recovered Ag NPs as recyclable material for environmental remediation.




Similar content being viewed by others
References
Alivisatos P (2004) The use of nanocrystals in biological detection. Nat Biotechnol 22:47–52
Chaloupka K, Malam Y, Seifalian AM (2010) Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol 28:580–588
Dror-Ehre A, Adin A, Markovich G, Mamane H (2010) Control of biofilm formation in water using molecularly capped silver nanoparticles. Water Res 44:2601–2609
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Li LXY, Hartmann G, Döblinger M, Schuster M (2013) Quantification of nanoscale silver particles removal and release from municipal wastewater treatment plants in Germany. Environ Sci Technol 47:7317–7323
Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY (2009) Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 5:1897–1910
AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S (2008) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290
Aline AB, Claudio MJ, Fernanda CP, Maria CS, Luiz HCM, Daniel SC, Marcos D (2015) Toxicity of PVA-stabilized silver nanoparticles to algae and microcrustaceans. Environ Nanotech Monit Manag 3:22–29
Loeschner K, Hadrup N, Qvortrup K, Larsen A, Gao X, Vogel U, Mortensen A, Lam HR, Larsen EH (2011) Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part Fibre Toxicol 8:18–23
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R (2008) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42:8959–8964
Arnaout CL, Gunsch CK (2012) Impacts of silver nanoparticle coating on the nitrification potential of nitrosomonas europaea. Environ Sci Technol 46:5387–5395
Khan SS, Mukherjee A, Chandrasekaran N (2012) Adsorptive removal of silver nanoparticles (SNPs) from aqueous solution by Aeromonas punctata and its adsorption isotherm and kinetics. Colloids Surf B 92:156–160
Dhandayuthapani B, Mallampati R, Sriramulu D, Dsouza RF, Valiyaveettil S (2014) PVA/gluten hybrid nanofibers for removal of nanoparticles from water. ACS Sustain Chem Eng 2:1014–1021
Mahanta N, Valiyaveettil S (2011) Surface modified electrospun poly(vinyl alcohol) membranes for extracting nanoparticles from water. Nanoscale 3:4625–4631
Sauceda IS, Ortega MMC, Munive GT, Castillo JMQ, Castro TDC, Romero MAE, Vega MA, Ramírez JZ, Castillo LSQ (2016) Selective adsorption of metallic complex using polyaniline or polypyrrole. Mater Chem Phys 182:39–48
Chowdhury AN, Jesmeen SR, Hossain MM (2004) Removal of dyes from water by conducting polymeric adsorbent. Polym Adv Technol 15:633–640
Guo X, Fei GT, Su H, Zhang LD (2011) Synthesis of polyaniline micro/nanospheres by a copper(II)-catalyzed self-assembly method with superior adsorption capacity of organic dye from aqueous solution. J Mater Chem 21:8618–8624
Mahanta D, Madras G, Radhakrishnan S, Patill S (2008) Adsorption of sulfonated dyes by polyaniline emeraldine salt and its kinetics. J Phys Chem B 112:10153–10160
Mahanta D, Madras G, Radhakrishnan S, Patill S (2009) Adsorption and desorption kinetics of anionic dyes on doped polyaniline. J Phys Chem B 113:2293–2299
Yan B, Chen ZH, Cai L, Chen ZM, Fu JW, Xu Q (2015) Fabrication of polyaniline hydrogel: synthesis, characterization and adsorption of methylene blue. Appl Surf Sci 356:39–47
Xu B, Wang YP, Jin R, Li XP, Song DQ, Zhang HQ, Sun Y (2015) Magnetic solid-phase extraction based on Fe3O4@polyaniline particles followed by ultrafast liquid chromatography for determination of Sudan dyes in environmental water samples. Anal Methods 7:1606–1614
Sun YP, Xiao F, Liu XG, Feng C, Jin CG (2013) Preparation and electromagnetic wave absorption properties of core–shell structured Fe3O4–polyaniline nanoparticles. RSC Adv 3:22554–22559
Bai HP, Zheng YP, Wang TY, Peng NK (2016) Magnetic solvent-free nanofluid based on Fe3O4/polyaniline nanoparticles and its adjustable electric conductivity. J Mater Chem A 4:14392–14399
Zhou QX, Wang YQ, Xiao JP, Fan HL (2016) Adsorption and removal of bisphenol A, α-naphthol and β-naphthol from aqueous solution by Fe3O4@polyaniline core–shell nanomaterials. Synth Met 5:113–122
Ma Y, Qiao MT, Chen YH, Hou CP, Zhang BL, Zhang QY (2015) Fabrication of electromagnetic Fe3O4@polyaniline nanofibers with high aspect ratio. RSC Adv 5:9986–9992
Maleki A, Movahed H, Ravaghi P, Kari T (2016) Facile in situ synthesis and characterization of a novel PANI/Fe3O4/Ag nanocomposite and investigation of catalytic applications. RSC Adv 6:98777–98787
Maleki A, Movahed H, Paydar R (2016) Design and development of a novel cellulose/γ-Fe2O3/Ag nanocomposite: a potential green catalyst and antibacterial agent. RSC Adv 6:13657–13665
Maleki A, Ravaghi P, Aghaei M (2017) A novel magnetically recyclable silver-loaded cellulose-based bionanocomposite catalyst for green synthesis of tetrazolo[1,5-a]pyrimidines. Res Chem Intermed 43:5485–5494
Maleki A (2018) Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason Sonochem 40:460–464
Maleki A (2012) Fe3O4/SiO2 nanoparticles: an efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron 68:7827–7833
Maleki A (2013) One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Lett 54:2055–2059
Qian K, Liu HL, Yang LB, Liu JH (2012) Designing and fabricating of surface-enhanced Raman scattering substrate with high density hot spots by polyaniline template-assisted self-assembly. Nanoscale 46:449–645
Xia YY, Li TJ, Ma C, Gao C, Chen J (2014) Au/montmorillonite/polyaniline nanoflakes: facile fabrication by self-assembly and application as catalyst. RSC Adv 4:20516–20520
Dhawan SK, Trivedi DC (1991) Electrochemical behaviour of polyaniline in aromatic sulphonic acids. Polym Int 25:55–61
Miroslava T, Eva T, Jaroslav S (2004) FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym Degrad Stab 86:179–185
Yang S, Nie C, Liu HR, Liu M (2003) Facile synthesis and catalytic application of Ag–Fe2O3–Carbons nanocompaosites. Mater Lett 100:296–298
Wang M, Tian D, Tian PP, Yuan LP (2013) Synthesis of micro-SiO2@nanoAg particles and their catalytic performance in 4-nitrophenol reduction. Appl Surf Sci 283:389–393
Shi Y, Zhang XL, Feng G, Chen XS, Lu ZH (2015) Ag-SiO2 nanocomposites with plum-pudding structure as catalyst for hydrogenation of 4-nitrophenol. Ceram Int 41:14660–14667
Yan MQ, ShenY Zhang GY, Bi H (2016) Multifunctional nanotube-like Fe3O4/PANI/CDs/Ag hybrids: an efficient SERS substrate and nanocatalyst. Mater Sci Eng. C 58:568–575
Acknowledgements
This study was funded by STRP of Anhui University of Technology (2016307986).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Q. Removal and reuse of Ag nanoparticles by magnetic polyaniline/Fe3O4 nanofibers. J Mater Sci 53, 8901–8908 (2018). https://doi.org/10.1007/s10853-018-2181-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2181-z