Abstract
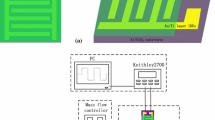
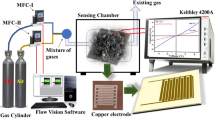
The control in morphology and microstructure is an efficient approach to regulate the gas sensing properties of the sensor. In this work, polyaniline (PANi) layer in nanoscale film morphology was coated on the surface of reduced graphene oxide (rGO) sheets through the in situ polymerization method. The microstructure and properties of the composite sheets were characterized by scanning electron microscopy, atomic force microscopy, Fourier transform infrared spectrometry, Raman spectrometry and thermal gravimetric analysis. It is found that there are strong effects of morphology-dependent structures of PANi layers on the sensing behaviors for ammonia detection, and more importantly, the utilization of the composite sheets as a new type of sensing material for highly sensitive detection of formaldehyde. Moreover, the best sensing properties can be achieved for the composite sheets with the thickness of PANi layer of 1.3 nm, benefiting from the strongest interaction between graphene sheet and PANi chains among all the composite sheets. Furthermore, a wide linear response range from 12 to 1500 ppm, 10 s response time for 100 ppm, 3.0 × 10−4/ppm sensitivity and 10 ppm detection limit can be achieved for ammonia, while 8 to 250 ppm, 35 s, 1.1 × 10−3/ppm and 4 ppm for formaldehyde. With the advantages in simplicity in material preparation and device operation as well as significant synergistic effects, PANi in nanoscale film morphology covered on the rGO sheets with large surface area can provide an efficient approach for fabricating sensitive and cheap chemiresistive sensor as an early warning system in some particular situations where either ammonia or formaldehyde is used widely or produced largely.








Similar content being viewed by others
References
Wang T, Huang D, Yang Z, Xu S, He G, Li X, Hu N, Yin G, He D, Zhang L (2016) A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano Micro Lett 8:95–119
Meng F, Guo Z, Huang X (2015) Graphene-based hybrids for chemiresistive gas sensors. TrAC-Trend. Anal Chem 68:37–47
Latif U, Dickert FL (2015) Graphene hybrid materials in gas sensing applications. Sensors 15:30504–30524
Varghese SS, Lonkar S, Singh KK, Swaminathan S, Abdala A (2015) Recent advances in graphene based gas sensors. Sens Actuators B 218:160–183
Fratoddi I, Venditti I, Cametti C, Russo MV (2015) Chemiresistive polyaniline-based gas sensors: a mini review. Sens Actuators B 220:534–548
Virji S, Huang J, Kaner RB, Weiller BH (2004) Polyaniline nanofiber gas sensors: examination of response mechanisms. Nano Lett 4:491–496
Stewart KME, McManus NT, Abdel-Rahman E, Penlidis A (2012) Doped polyaniline for the detection of formaldehyde. J Macromol Sci A 49:1–6
Matsuguchi M, Asahi T (2011) Properties and stability of polyaniline nanofiber ammonia sensors fabricated by novel on-substrate method. Sens Actuators B 160:999–1004
Kukla AL, Shirshov YM, Piletsky SA (1996) Ammonia sensors based on sensitive polyaniline films. Sens Actuators B 37:135–140
Ariyageadsakul P, Vchirawongkwin V, Kritayakornupong C (2016) Determination of toxic carbonyl species including acetone, formaldehyde, and phosgene by polyaniline emeraldine gas sensorusing DFT calculation. Sens Actuators B 232:165–174
Sen T, Mishra S, Shimpi NG (2016) Synthesis and sensing applications of polyaniline nanocomposites: a review. RSC Adv 6:42196–42222
Konwer S, Guha AK, Dolui SK (2013) Graphene oxide-filled conducting polyaniline composites as methanol-sensing materials. J Mater Sci 48:1729–1739. https://doi.org/10.1007/s10853-012-6931-z
Bai S, Zhao Y, Sun J, Tian Y, Luo R, Li D, Chen A (2015) Ultrasensitive room temperature NH3 sensor based on a graphene–polyaniline hybrid loaded on PET thin film. Chem Commun 51:7524–7527
Ponnamma D, Guo Q, Krupa I, Al-Maadeed MASA, Varughese KT, Thomas S, Sadasivuni K (2015) Graphene and graphitic derivative filled polymer composites as potential sensors. Phys Chem Chem Phys 17:3954–3981
Valles C, Jimenez P, Munoz E, Benito AM, Maser WK (2011) Simultaneous reduction of graphene oxide and polyaniline: doping-assisted formation of a solid-state charge-transfer complex. J Phys Chem C 115:10468–10474
Gavgania JN, Hasani A, Nouri M, Mahyari M, Salehi A (2016) Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens Actuators B 229:239–248
Tripathi KM, Kim T, Losic D, Tung TT (2016) Recent advances in engineered graphene and composites for detection of volatile organic compounds (VOCs) and non-invasive diseases diagnosis. Carbon 110:97–129
Konwer S, Begum A, Bordoloi S, Boruah R (2017) Expanded graphene-oxide encapsulated polyaniline composites as sensing material for volatile organic compounds. J Polym Res 24:37
Gaikwad G, Patil P, Patil D, Naik J (2017) Synthesis and evaluation of gas sensing properties of PANI based graphene oxide nanocomposites. Mat Sci Eng B 218:14–22
Huang X, Hu N, Gao R, Yu Y, Wang Y, Yang Z, Kong ES, Wei H, Zhang Y (2012) Reduced graphene oxide–polyaniline hybrid: preparation, characterization and its applications for ammonia gas sensing. J Mater Chem 22:22488–22495
Wu Z, Chen X, Zhu S, Zhou Z, Yao Y, Quan W, Liu B (2013) Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens Actuators B 178:485–493
Wu Z, Chen X, Zhu S, Zhou Z, Yao Y, Quan W, Liu B (2013) Room temperature methane sensor based on graphene nanosheets/polyaniline nanocomposite thin film. IEEE Sens J 13:777–782
Huang X, Hu N, Zhang L, Wei L, Wei H, Zhang Y (2013) The NH3 sensing properties of gas sensors based on aniline reduced graphene oxide. Synth Metals 185:25–30
Guo Y, Wang T, Chen F, Sun X, Li X, Yu Z, Wan P, Chen X (2016) Hierarchical graphene–polyaniline nanocomposite films for high–performance flexible electronic gas sensors. Nanoscale 8:12073–12080
Antwi-Boampong S, BelBruno JJ (2013) Detection of formaldehyde vapor using conductive polymer films. Sens Actuators B 182:300–306
Qin X, Meng Q, Zhao W (2011) Effects of Stone-Wales defect upon adsorption of formaldehyde on graphene sheet with or without Al dopant: a first principle study. Surf Sci 605:930–933
Liang B, Qin Z, Li T, Dou Z, Zeng F, Cai Y, Zhu M, Zhou Z (2015) Poly (aniline-co-pyrrole) on the surface of reduced graphene oxide as high-performance electrode materials for supercapacitors. Electrochim Acta 177:335–342
Duan Y, Liu J, Zhang Y, Wang T (2016) First–principles calculations of graphene-based polyaniline nano-hybrids for insight of electromagnetic properties and electronic structures. RSC Adv 6:73915–73923
Wang R, Huang L, Tian X (2012) Understanding the protonation of polyaniline and polyaniline–graphene interaction. J Phys Chem C 116:13120–13126
Hu N, Yang Z, Wang Y, Zhang L, Wang Y, Huang X, Wei H, Wei L, Zhang Y (2014) Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology 25:025502
Zhang X, Qin Z, Liu X, Liang B, Liu N, Zhou Z, Zhu M (2013) Flexible sensing fibers based on polyaniline-coated polyurethane for chloroform vapor detection. J Mater Chem A 1:10327–10333
Deore B, Diaz-Quijada GA, Wayner DDM, Stewart D, Won DY, Waldron P (2011) An electronic nose for the detection of carbonyl species. ECS Trans 35:83–88
Acknowledgement
The authors gratefully acknowledge financial supports from the National Natural Science Foundation of China (21274019). Prof. Zhou also thanks the financial supports from the Hunan Provincial Education Department Foundation (15C1366) and Xiangxi National Vocational–Technical College (K201601).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ding, L., Qin, Z., Dou, Z. et al. Morphology-promoted synergistic effects on the sensing properties of polyaniline ultrathin layers on reduced graphene oxide sheets for ammonia and formaldehyde detection. J Mater Sci 53, 7595–7608 (2018). https://doi.org/10.1007/s10853-018-2109-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2109-7