Abstract
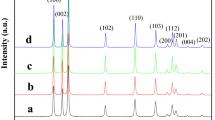
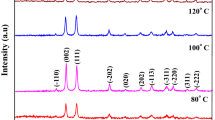
ZnO nanostructures with divergent morphologies were synthesized by a facile chemical approach using various solvents and reducing agents. The synthesized nanostructures were characterized by XRD, electron microscopic techniques and fluorescence spectroscopy. The microstructural analysis shows that different morphologies of ZnO can be formed when suitable reducing agents (RAs) and solvents are used with variation in the molar ratio (β) of zinc precursor to RAs. The morphologies observed are spindles, flowers, nanoassemblies, rods and hexagonal hollow tubes. The size of spindle-shaped ZnO nanostructures varies from 1 to 5 μm, and nanoassemblies are of around 150 ± 20 nm. Each nanoassembly is the aggregation of individual nanoparticles of sizes around 4–5 nm. The optical study shows that these ZnO nanostructures have various defect concentrations and surface properties. Furthermore, the alterations and transformations in the physical and chemical properties of ZnO nanostructures significantly influenced the photo-catalytic degradation of methylene blue (MB) dye under solar irradiation. A complete degradation of MB is observed within 130 and 60 min when treated with ZnO of suitable morphology under UV and solar light radiation. This variation in time may be attributed to the availability of higher specific surface area, large amount of defects and anisotropy in morphology.







Similar content being viewed by others
References
Bhuyan B, Paul B, Purkayastha DD, Dhar SS, Behera S (2016) Facile synthesis and characterization of zinc oxide nanoparticles and studies of their catalytic activity towards ultrasound-assisted degradation of metronidazole. Mater Lett 168:158–162. doi:10.1016/j.matlet.2016.01.024
Drmosh QA, Yamani ZH (2016) Synthesis, characterization, and hydrogen gas sensing properties of AuNs-catalyzed ZnO sputtered thin films. Appl Surf Sci 375:57–64. doi:10.1016/j.apsusc.2016.02.238
Kaur M, Kailasaganapathi S, Ramgir N (2017) Gas dependent sensing mechanism in ZnO nanobelt sensor. Appl Surf Sci 394:258–266. doi:10.1016/j.apsusc.2016.10.085
Sehgal P, Narula AK (2016) Quantum dot cosensitized solar cell based on PMOT@CdSe@ZnO core shell nanostructures with dual emission. J Solid State Chem 233:428–437. doi:10.1016/j.jssc.2015.11.013
Singh S, Barick KC, Bahadur D (2013) Shape-controlled hierarchical ZnO architectures: photocatalytic and antibacterial activities. CrystEngComm 15:4631–4639. doi:10.1039/C3CE27084J
Gupta J, Bhargava P, Bahadur D (2015) Fluorescent ZnO for imaging and induction of DNA fragmentation and ROS-mediated apoptosis in cancer cells. J Mater Chem B 3:1968–1978. doi:10.1039/C4TB01661K
Prakash A, Bahadur D (2014) The role of ionic electrolytes on capacitive performance of ZnO-reduced graphene oxide nanohybrids with thermally tunable morphologies. ACS Appl Mater Interfaces 6:1394–1405. doi:10.1021/am405031y
Hong RY, Li JH, Chen LL (2009) Synthesis, surface modification and photocatalytic property of ZnO nanoparticles. Powder Technol 189:426–432. doi:10.1016/j.powtec.2008.07.004
Ungul J, Dejene BF (2016) Effect of solvent medium on the structural, morphological and optical properties of ZnO nanoparticles synthesized by the sol–gel method. Phys B 480:26–30. doi:10.1016/j.physb.2015.10.007
Gupta J, Barick KC, Bahadur D (2011) Defect mediated photocatalytic activity in shape-controlled ZnO nanostructures. J Alloys Compds 509:6725–6730. doi:10.1016/j.jallcom.2011.03.157
Debbarma M, Das S, Saha M (2013) Effect of reducing agents on the structure of zinc oxide under microwave irradiation. Adv Manuf 1:183–186. doi:10.1007/s40436-013-0020-7
Xie J, Li Y, Zhao W, Bian L, Wei Y (2011) Simple fabrication and photocatalytic activity of ZnO particles with different morphologies. Powder Technol 207:140–144. doi:10.1016/j.powtec.2010.10.019
Panigrahy B, Aslam M, Misra DS, Bahadur D (2009) Polymer-mediated shape-selective synthesis of ZnO nanostructures using a single-step aqueous approach. CrystEngComm 11:1920–1925. doi:10.1039/B904833M
Gupta J, Bhargava P, Bahadur D (2014) Morphology dependent photocatalytic and magnetic properties of ZnO nanostructures. Phys B 448:16–19. doi:10.1016/j.physb.2014.03.081
Wang H, Xie C, Zhang W, Cai S, Yang Z, Gui Y (2007) Comparison of dye degradation efficiency using ZnO powders with various size scales. J Hazard Mater 141:645–652. doi:10.1016/j.jhazmat.2006.07.021
Tong G-X, Du F-F, Liang Y (2013) Polymorphous ZnO complex architectures: selective synthesis, mechanism, surface area and Zn-polar plane-codetermining antibacterial activity. J Mater Chem B 1:454–463. doi:10.1039/C2TB00132B
Hong Y, Wang J, Yuan B (2014) Template free synthesis of ZnO spindles and flowers via hydrothermal route. Adv Appl Ceram 113:178–183. doi:10.1179/1743676113Y.0000000139
Xiong H-M, Ma R-Z, Wang S-F, Xia Y-Y (2011) Photoluminescent ZnO nanoparticles synthesized at the interface between air and triethylene glycol. J Mater Chem 21:3178–3182. doi:10.1039/C0JM02577A
Yang C, Li Q, Tang L, Bai A, Song H, Yu Y (2016) Monodispersed colloidal zinc oxide nanospheres with various size scales: synthesis, formation mechanism, and enhanced photocatalytic activity. J Mater Sci 51:5445–5459. doi:10.1007/s10853-016-9848-0
Lee S, Jeong S, Kim D, Hwang S, Jeon M, Moon J (2008) ZnO nanoparticles with controlled shapes and sizes prepared using a simple polyol synthesis. Superlattices Microstruct 43:330–339. doi:10.1016/j.spmi.2008.01.004
Romero R, Leinen D, Dalchiele EA, Ramos-Barrado JR, Martín F (2006) The effects of zinc acetate and zinc chloride precursors on the preferred crystalline orientation of ZnO and Al-doped ZnO thin films obtained by spray pyrolysis. Thin Solid Films 515:1942–1949. doi:10.1016/j.tsf.2006.07.152
Liu B, Zeng HC (2004) Room temperature solution synthesis of monodispersed single-crystalline ZnO nanorods and derived hierarchical nanostructures. Langmuir 20:4196–4204. doi:10.1021/la035264o
Panigrahy B, Aslam M, Misra DS, Ghosh M, Bahadur D (2010) Defect-related emissions and magnetization properties of ZnO nanorods. Adv Funct Mater 20:1161–1165. doi:10.1002/adfm.200902018
Andelman T, Gong Y, Polking M (2005) Morphological control and photoluminescence of zinc oxide nanocrystals. J Phys Chem B 109:14314–14318. doi:10.1021/jp050540o
Klubnuan S, Suwanboon S, Amornpitoksuk P (2016) Effects of optical band gap energy, band tail energy and particle shape on photocatalytic activities of different ZnO nanostructures prepared by a hydrothermal method. Opt Mater 53:134–141. doi:10.1016/j.optmat.2016.01.045
Sharma A, Singh BP, Dhar S, Gondorf A, Spasova M (2012) Effect of surface groups on the luminescence property of ZnO nanoparticles synthesized by sol–gel route. Surf Sci 606:L13–L17. doi:10.1016/j.susc.2011.09.006
Li H, Schirra LK, Shim J (2012) Zinc oxide as a model transparent conducting oxide: a theoretical and experimental study of the impact of hydroxylation, vacancies, interstitials, and extrinsic doping on the electronic properties of the polar ZnO (0002) surface. Chem Mater 24:3044–3055. doi:10.1021/cm301596x
Bhattacharjee R, Hung IM (2014) Effect of different concentration Li-doping on the morphology, defect and photovoltaic performance of Li–ZnO nanofibers in the dye-sensitized solar cells. Mater Chem Phys 143:693–701. doi:10.1016/j.matchemphys.2013.09.055
Wang J, Mei Y, Lu X (2016) Effects of annealing pressure and Ar+ sputtering cleaning on Al-doped ZnO films. Appl Surf Sci 387:779–783. doi:10.1016/j.apsusc.2016.06.069
Patra MK, Manoth M, Singh VK (2009) Synthesis of stable dispersion of ZnO quantum dots in aqueous medium showing visible emission from bluish green to yellow. J Lumin 129:320–324. doi:10.1016/j.jlumin.2008.10.014
Sudha M, Senthilkumar S, Hariharan R, Suganthi A, Rajarajan M (2013) Synthesis, characterization and study of photocatalytic activity of surface modified ZnO nanoparticles by PEG capping. J Sol-Gel Sci Technol 65:301–310. doi:10.1007/s10971-012-2936-y
Liu X, Chen N, Xing X (2015) A high-performance n-butanol gas sensor based on ZnO nanoparticles synthesized by a low-temperature solvothermal route. RSC Adv. 5:54372–54378. doi:10.1039/C5RA05148G
Barick KC, Aslam M, Dravid VP, Bahadur D (2008) Self-aggregation and assembly of size-tunable transition metal doped ZnO nanocrystals. J Phys Chem C 112:15163–15170. doi:10.1021/jp802361r
Ding J, Fang X, Yang R, Kan B, Li X, Yuan N (2014) Transformation of ZnO polycrystalline sheets into hexagon-like mesocrystalline ZnO rods (tubes) under ultrasonic vibration. Nanoscale Res Lett 9:214–219. doi:10.1186/1556-276X-9-214
Feng J-J, Wang Z-Z, Li Y-F, Chen J-R, Wang A-J (2013) Control growth of single crystalline ZnO nanorod arrays and nanoflowers with enhanced photocatalytic activity. J Nanopart Res 15:1565–1577. doi:10.1007/s11051-013-1565-x
Kathalingam A, Park H-C, Kim S-D, Kim H-S, VelumaniS Mahalingam T (2015) Synthesis of ZnO nanorods using different precursor solutions and their two terminal device characterization. J Mater Sci Mater Electron 26:5724–5734. doi:10.1007/s10854-015-3129-6
Barick KC, Sharma P, Mukhija A, Sainis JK, Gupta A, Hassan PA (2015) Effect of cetylpyridinium chloride on surface passivation and photocatalytic activity of ZnO nanostructures. J Environ Chem Eng 3:1346–1355. doi:10.1016/j.jece.2014.12.007
Flores NM, Pal U, Galeazzi R, Sandoval A (2014) Effects of morphology, surface area, and defect content on the photocatalytic dye degradation performance of ZnO nanostructures. RSC Adv 4:41099–41110. doi:10.1039/C4RA04522J
Zhang X, Qin J, Xue Y, Yu P, Zhang B, Wang L, Liu R (2014) Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci Rep 4:4596–4603. doi:10.1038/srep04596
Barhoum A, Melcher J, Van Assche G (2017) Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: porous microparticles versus nonporous nanoparticles. J Mater Sci 52:2746–2762. doi:10.1007/s10853-016-0567-3
Ullah R, Dutta J (2008) Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J Hazard Mater 156:194–200. doi:10.1016/j.jhazmat.2007.12.033
Kaviya S, Prasad E (2016) Eco-friendly synthesis of ZnO nanopencils in aqueous medium: a study of photocatalytic degradation of methylene blue under direct sunlight. RSC Adv 6:33821–33827. doi:10.1039/C6RA04306B
Henrique SO, Luiz CAO, Marcio CP (2015) Nanostructured vanadium-doped iron oxide: catalytic oxidation of methylene blue dye. J Chem 39:3051–3058. doi:10.1039/c4nj02063d
Wladmir FdS, Iara RG, Luiz CAO (2007) Natural and H2-reduced limonite for organic oxidation by a Fenton-like system: Mechanism study via ESI-MS and theoretical calculations. J Mol Catal A Chem 278:145–151. doi:10.1016/j.molcata.2007.09.003
Acknowledgements
The authors acknowledge the help of Sophisticated Analysis Instruments Facility, IIT Bombay, for providing SEM and TEM facilities. The financial support by Nanomission of DST, Government of India, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thongam, D.D., Gupta, J., Sahu, N.K. et al. Investigating the role of different reducing agents, molar ratios, and synthesis medium over the formation of ZnO nanostructures and their photo-catalytic activity. J Mater Sci 53, 1110–1122 (2018). https://doi.org/10.1007/s10853-017-1587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1587-3