Abstract
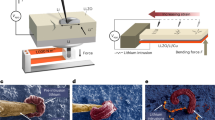
Lithiation/de-lithiation cycles induced cracks in isostatically pressed graphite samples subjected to constant-load bending tests while simultaneously conducting cyclic voltammetry (CV) experiments in propylene carbonate-based Li-ion battery electrolyte solutions. A large cyclic strain of Δε = 0.95% was generated by lithiation/de-lithiation cycles as determined by micro-Raman measurements carried out concurrently with CV experiments. In-situ optical microscopy measurements of crack lengths, a, showed that the crack-growth rate, da/dt, depended on the stress intensity factor at the crack tip and could be expressed as da/dt = AΔK nI . A two-stage crack-growth behaviour was determined with n = 51.3 in the first stage. However, lower crack propagation rates observed in the second stage (n = 9.9) were affected by crack closure due to (1) a rough fracture surface morphology of the graphite cracks, and (2) the deposition of solid electrolyte reduction products on facetted crack surfaces, where both the factors likely contributed to reducing ΔK to ΔK eff.









Similar content being viewed by others
References
Chung GC, Kim HJ, Yu SI, Jun SH, Choi JW, Kim MH (2000) Origin of graphite exfoliation—an investigation of the important role of solvent cointercalation. J Electrochem Soc 147:4391–4398
Aurbach D, Zinigrad E, Cohen Y, Teller H (2002) A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion 148:405–416
Cheng YT, Verbrugge MW (2010) Application of Hasselman’s crack propagation model to insertion electrodes. Electrochem Solid State 13:A128–A131
Bhattacharya S, Riahi AR, Alpas AT (2011) A transmission electron microscopy study of crack formation and propagation in electrochemically cycled graphite electrode in lithium-ion cells. J Power Sources 196:8719–8727
Woodford WH, Chiang YM, Carter WC (2010) “Electrochemical shock” of intercalation electrodes: a fracture mechanics analysis. J Electrochem Soc 157:A1052–A1059
Harris SJ, Deshpande RD, Qi Y, Dutta I, Cheng YT (2010) Mesopores inside electrode particles can change the Li-ion transport mechanism and diffusion-induced stress. J Mater Res 25:1433–1440
Bhattacharya S, Riahi AR, Alpas AT (2011) In-situ observations of lithiation/de-lithiation induced graphite damage during electrochemical cycling. Scripta Mater 64:165–168
Aurbach D, Koltypin M, Teller H (2002) In situ AFM imaging of surface phenomena on composite graphite electrodes during lithium insertion. Langmuir 18:9000–9009
Zhang HL, Li F, Liu C, Tan J, Cheng HM (2005) New insight into the solid electrolyte interphase with use of a focused ion beam. J Phys Chem B 109:22205–22211
Liu P, Wang J, Hicks-Garner J, Sherman E, Soukiazian S, Verbrugge M, Tataria H, Musser J et al (2010) Aging mechanisms of LiFePO4 batteries deduced by electrochemical and structural analyses. J Electrochem Soc 157:A499–A507
Bhattacharya S, Alpas AT (2012) Micromechanisms of solid electrolyte interphase formation on electrochemically cycled graphite electrodes in lithium-ion cells. Carbon 50:5359–5371
Sethuraman VA, Hardwick LJ, Srinivasan V, Kostecki R (2010) Surface structural disordering in graphite upon lithium intercalation/deintercalation. J Power Sources 195:3655–3660
Kostecki R, McLarnon F (2003) Microprobe study of the effect of Li intercalation on the structure of graphite. J Power Sources 119:550–554
Markervich E, Salitra G, Levi MD, Aurbach D (2005) Capacity fading of lithiated graphite electrodes studied by a combination of electroanalytical methods, Raman spectroscopy and SEM. J Power Sources 146:146–150
Qi Y, Harris SJ (2010) In Situ Observation of strains during lithiation of a graphite electrode. J Electrochem Soc 157:A741–A747
Bhattacharya S, Riahi AR, Alpas AT (2014) Electrochemical cycling behaviour of lithium carbonate (Li2CO3) pre-treated graphite anodes—SEI formation and graphite damage mechanisms. Carbon 77:99–112
Aurbach D, Markovsky B, Weissman I, Levi E, Ein-Eli Y (1999) On the correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim Acta 45:67–86
Suresh S, Zamiski GF, Ritchie RO (1981) Oxide-induced crack closure—an explanation for near-threshold corrosion fatigue crack growth-behavior. Metall Trans A 12:1435–1443
Renganathan S, Sikha G, Santhanagopalan S, White RE (2010) Theoretical analysis of stresses in a lithium ion cell. J Electrochem Soc 157(2):A155–A163
Sethuraman VA, Van Winkle N, Abraham DP, Bower AF, Guduru PR (2012) Real-time stress measurements in lithium-ion battery negative-electrodes. J Power Sources 206:334–342
Mukhopadhyay A, Tokranov A, Sena K, Xiao X, Sheldon BW (2011) Thin film graphite electrodes with low stress generation during Li-intercalation. Carbon 49:2742–2749
Mukhopadhyay A, Tokranov A, Xiao X, Sheldon BW (2012) Stress development due to surface processes in graphite electrodes for Li-ion batteries: a first report. Electrochim Acta 66:28–37
Aurbach D, Koltypin M, Teller H (2002) In situ AFM imaging of surface phenomena on composite graphite electrodes during lithium insertion. Langmuir 18:9000–9009
Zhao H, Park S-J, Shi F, Fu Y, Battaglia V, Ross PN, Liu G (2014) Propylene carbonate (PC)-based electrolytes with high coulombic efficiency for lithium-ion batteries. J Electrochem Soc 161(1):A194–A200
Zhang H-L, Sun C-H, Li F, Liu C, Tan J, Cheng H-M (2007) New insight into the interaction between propylene carbonate-based electrolytes and graphite anode material for lithium ion batteries. J Phys Chem C 111:4740–4748
Sheldon BW, Tokranov A (2016) Internal stress due to solvent co-intercalation in graphite electrodes for Li ion batteries. Extreme Mech Lett 9:379–385
ASTM-C1421: Standard test methods for determination of fracture toughness of advanced ceramics at ambient temperature (2016). ASTM International
Soltesz U, Richter H (1984) Mechanical behavior of selected ceramics. In: Ducheyne P, Hastings GW (eds) Metals and ceramics biomaterials, vol. 2, strength and surface. CRC Press, Boca Raton, pp 23–61
Hodkinson PH, Nadeau JS (1975) Slow crack growth in graphite. J Mater Sci 10:846–856. doi:10.1016/j.dental.2010.10.025
Ritchie RO, Dauskardt RH, Yu WK, Brendzel AM (1990) Cyclic fatigue-crack propagation, stress-corrosion, and fracture-toughness behavior in pyrolytic carbon-coated graphite for prosthetic heart-valve applications. J Biomed Mater Res 24:189–206
Reich S, Jantoljak H, Thomsen C (2000) Shear strain in carbon nanotubes under hydrostatic pressure. Phys Rev B 61:13389–13392
Thomsen C, Reich S, Ordejon P (2002) Ab initio determination of the phonon deformation potentials of graphene. Phys Rev B 65:073403
Hanfland M, Beister H, Syassen K (1989) Graphite under pressure—equation of state and 1st-order Raman modes. Phys Rev B 39:12598–12603
Panitz JC, Joho F, Novak P (1999) In situ characterization of a graphite electrode in a secondary lithium-ion battery using Raman microscopy. Appl Spectrosc 53:1188–1199
Broek D (1973) Artificial slow crack growth under constant stress. The r-curve concept in plane stress, In: Engineering Fracture Mechanics, Vol. 5, pp. 45–53
Fazluddin S (2002) Crack growth resistance in nuclear graphite. Ph.D. dissertation, University of Leeds
Hodgkins A, Marrow TJ, Mummery P, Marsden B, Fok A (2006) X-ray tomography observation of crack propagation in nuclear graphite. Mater Sci Technol Ser 22:1045–1051
Ouagne P, Neighbour GB, McEnaney B (2002) Crack growth resistance in nuclear graphites. J Phys D Appl Phys 35:927–934
Chi SH (2016) Comparison of fracture toughness (KIc) and strain energy release rate (G) of selected nuclear graphites. J Nucl Mater 476:188–197
Kim NH, Kuila T, Kim KM, Nahm SH, Lee JH (2012) Material selection windows for hybrid carbons/poly(phenylene sulfide) composite for bipolar plates of fuel cell. Polym Test 31:537–545
Harris SJ, Lu P (2013) Effects of inhomogeneities-nanoscale to mesoscale-on the durability of Li-ion batteries. J Phys Chem C 117(13):6481–6492
Bhattacharya S, Shafiei M, Alpas AT (2015) Microstructural characterization of nanocrystalline Sn-coated carbon fibre electrodes cycled in Li-ion cells. Metall Mater Trans E 2:208–219
Mayes IC, Baker TJ (1981) Load transference across crack faces during fatigue crack-growth at positive values of R in low growth-rate regime. Met Sci 15:320–322
Walker N, Beevers CJ (1979) Fatigue crack closure mechanism in titanium. Fatigue Eng Mater 1:1460–2695
Acknowledgements
The authors thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for providing financial support in the form of a Discovery Grant.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
According to ASTM-C1421 [27] for four-point flexure with 0.35 ≤ a≤0.60, K I (or K max) can be expressed as,
in which, P is the load applied, a is the crack length measured by in-situ optical microscopy, B is the thickness of the test specimen measured perpendicular to a, W is the width measured parallel to a and f is a function of the ratio a/W for four-point flexure expressed as,
Rights and permissions
About this article
Cite this article
Sun, G., Bhattacharya, S. & Alpas, A.T. Cyclic strain-induced crack growth in graphite during electrochemical testing in propylene carbonate-based Li-ion battery electrolytes. J Mater Sci 53, 1297–1309 (2018). https://doi.org/10.1007/s10853-017-1547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1547-y