Abstract
Kesterite, Cu2ZnSnS4 (CZTS), is a promising absorber layer for use in photovoltaic cells. We report the use of copper, zinc and tin xanthates in melt reactions to produce Cu2ZnSnS4 (CZTS) thin films. The phase of the as-produced CZTS is dependent on decomposition temperature. X-ray diffraction patterns and Raman spectra show that films annealed between 375 and 475 °C are tetragonal, while at temperatures <375 °C hexagonal material was obtained. The electrical parameters of the CZTS films have also been determined. The conduction of all films was p-type, while the other parameters differ for the hexagonal and tetragonal materials: resistivity (27.1 vs 1.23 Ω cm), carrier concentration (2.65 × 10+15 vs 4.55 × 10+17 cm−3) and mobility (87.1 vs 11.1 cm2 V−1 s−1). The Hall coefficients were 2.36 × 103 versus 13.7 cm3 C−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper zinc tin sulfide (Cu2ZnSnS4, CZTS) is a promising absorber layer for use in photovoltaic cells and is composed of low toxicity [1,2,3] and earth-abundant elements [4,5,6]. It has a large absorption coefficient (α ≥ 104 cm−1) and a direct band gap of about 1.45 eV [7,8,9,10]. The performance of CZTS absorber layers is still less than that of silicon [11], which is the current industrial standard; however, unlike silicon, it has the advantage of a direct and tunable band gap [12].
CZTS thin films have been prepared using several techniques, including: chemical vapor deposition (CVD) [13], co-sputtering followed by sulfurization from the vapor phase [14], chemical bath deposition (CBD) [15], successive ionic layer adsorption and reaction (SILAR) [16] and solvothermal treatment of a layered elemental (copper, zinc and tin) film with sulfur powder [17]. However, there are two major problems with routes that involve the deposition of the individual components (either as metal M or M x S y ) followed by high-temperature annealing in a sulfur atmosphere. The first issue is the loss of volatile components such as SnS. The photoelectrical performance of CZTS is highly dependent on good stoichiometric control, and such evaporation can make it difficult to control the composition of the target phase [18,19,20,21,22]. The second issue is the stability of the Mo electrode that the CZTS is often deposited on; this can react with sulfur to form a MoS2 layer between the electrode and the CZTS, resulting in a dramatic decrease in performance of the photovoltaic device [23, 24]. These two problems indicate a requirement for a new synthetic route to CZTS that avoids higher temperatures.
The Cu2ZnSnS4 unit cell is based on zinc blende: It is related to the chalcopyrite structure of CuInS2 by changing indium for zinc and tin, with the metals in tetrahedral coordination [25]. There are three different phases of CZTS: kesterite, stannite and primitive mixed CuAu-like structure (PMCA) [26,27,28]. Zunger et al. [29, 30] have established that strain energy is responsible for the stability of the CZTS crystal structure. They found that for large lattice mismatches, the kesterite structure has a lower strain energy, which means that chalcopyrite is more stable than the CuAu-like structure; as a result, the stability of kesterite is higher than that of stannite and PMCA, but there is little difference in strain energy between the kesterite and stannite structures (~3 meV/atom).
Walsh et al. have performed calculations on the total energy of CZTS and other quaternary semiconductors of the form I2-II-IV-VI4 (I=Cu, Ag; II=Zn, Cd; IV=Si, Ge, Sn; VI=S, Se). They found that the kesterite structure is thermodynamically more stable than stannite, wurtzite-kesterite and wurtzite-stannite [31,32,33]. In stannite, (Cu/Fe)Sn layers alternate with Cu2 layers in the unit cell, whereas for kesterite CuSn alternates with CuZn. The similar lattice constants and total energy values for kesterite and stannite CZTS mean that there is often the possibility of both phases existing together depending on the methods used to prepare the material [34].
A big disadvantage of CZTS in photovoltaic applications is its ability to crystallize in these different forms. Schorr et al. [35] synthesized tetragonal CZTS at 860 °C and the cubic phase of CZTS at temperatures >885 °C by the solid-state reaction of the pure elements in sealed evacuated silica tubes. Brandl et al. [36] synthesized CZTS nanoparticles in a disordered cubic structure by the hot injection of CuCl2, Zn(OAc)2 and SnCl2 with S in oleylamine at 225 °C, and they found that a disordered cubic phase changed to the tetragonal CZTS phase at 275 °C. Cattley et al. [37] have also observed a temperature-dependent phase change from tetragonal kesterite to hexagonal wurtzite during their synthesis of quantum dots from Cu(acetylacetonate)2, Zn(OAc)2, SnCl4 and S(SiMe3)2. Nakayama et al. [38] first successfully prepared stannite CZTS thin film via spray pyrolysis, and its electronic properties have been theoretically assessed by Marques et al. [39] and Scarpulla et al. [40]. The development of a method for controlling the phase of the obtained CZTS would be a major step toward commercializing CZTS. We have previously reported the synthesis of CZTS and Cu2Zn1−xFexSnS4 nanoparticles and thin films from the decomposition of dithiocarbamate single-source precursors (SSPs) [41,42,43].
Recently xanthates have been used as SSPs to metal sulfide nanocrystals [44,45,46,47,48,49]. They have the general chemical formula [M(S2COR)n], where R is an alkyl group. Xanthates are good precursors to deposit metal sulfide thin films as the preformed M–S bonds make the conversion to a metal sulfide film straightforward [50, 51]. They can also decompose at lower temperatures compared to other precursors, and they are usually held to decompose by the Chugaev elimination reaction [52]. A range of xanthates and a parallel range of dithiocarbamates have been synthesized by Molloy and co-workers as sources of metal sulfides. They studied thermal decomposition profiles by thermogravimetric analysis (TGA), and their experiments show that metal xanthates are viable precursors for Cu2ZnSnS4, in both thin film and nanoparticulate form [53].
In this report, we discuss the synthesis of a range of copper, zinc and tin O-ethyl and O-n-butylxanthates and assessed their suitability as precursors to CZTS. On the basis of decomposition properties [(Ph3P)2CuS2COEt], [Zn(S2COnBu)2] and [Sn(S2COEt)2] have been used as coating precursors for the production of Cu2ZnSnS4 films on glass. We focus on both the annealing temperature and the role of the xanthate ligand in the decomposition process for the potential in control of the structural and electronic properties of the CZTS films produced.
The CZTS films were analyzed by powder X-ray diffraction (p-XRD), Raman spectroscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM). We investigated the electrical properties using the van der Pauw method [54], and the resistivity of the films was calculated from the Hall voltage and sheet resistance.
Experimental
All chemicals, with the exception of tin(II) chloride (Alfa Aesar), were purchased from Sigma-Aldrich and were used as received. Elemental analysis (EA) and thermogravimetric analysis (TGA) were carried out by the Microelemental Analysis service at the University of Manchester. EA was performed using a Flash 2000 Thermo Scientific elemental analyzer, and TGA data were obtained with Mettler-Toledo TGA/DSC1 stare system between the range of 30–600 °C at a heating rate of 10 °C min−1 under nitrogen flow. Scanning electron microscopy (SEM) images were obtained using a Philips XL30 FEG, with energy-dispersive X-ray spectroscopy (EDX) data obtained using a DX4 instrument. Samples suitable for transmission electron microscopy (TEM) were prepared by exfoliating thin films in toluene and dropcasting the suspension onto holey carbon support grids, which were then air-dried. TEM was performed using Philips CM20 equipped with a LaB6 source (Fig. 5a, b) or a FEI Tecnai G2 F30 with Schottky field emitter operated at 300 keV (Fig. 5c). Powder X-ray diffraction (p-XRD) analyses were carried out using an X’pert diffractometer with a Cu–K α1 source (λ = 1.54059 Å), the samples were scanned between 20 and 75°, and the applied voltage was 40 kV and the current 30 mA. Raman spectra were measured using a Renishaw 1000 Micro-Raman System equipped with a 514 nm laser.
Synthesis of metal xanthate complexes
Synthesis of potassium n-butylxanthate ligand
The synthesis of [K(S2COnBu)] was adapted from a literature procedure [55]. KOH (5.64 g, 0.10 mol) and nBuOH (50 ml) were stirred for 2 h at room temperature, and then, CS2 (7.73 g, 6.11 ml, 0.10 mol) was added dropwise to the reaction, resulting in an orange solution. The unreacted alcohol was removed in vacuo, and the yellow solid product was dried and recrystallized from n-butyl alcohol to give [K(S2COnBu)] (13.45 g, 71.5 mmol, 71.5% yield). MPt: 232–235 °C.
Calc. for C5H9KOS2 (%): C 31.9, H 4.82, S 34.0, K 21.8; found: C 31.6, H 4.51, S 33.3, K 22.0.
FT-IR (cm−1): 2958 (m), 2869 (w), 1461 (m). 1445 (w), 1261 (s), 1149 (m), 1173 (m), 1062 (m), 1014 (m), 747.3 (m), 669.0 (s), 566.2 (s).
Synthesis of bis(O-butylxanthato)zinc(II)
The complex [Zn(S2COnBu)2] was synthesized by a similar method to [Zn(S2COEt)2] using [K(S2COnBu)] (5.00 g, 0.027 mol) and ZnCl2 (1.81 g, 0.013 mol). Yield = 4.61 g, 0.013 mol, 97%. MPt: 105–112 °C.
Calc. for C10H18O2S4Zn (%): C 33.0, H 4.99, S 35.2, Zn, 18.0; found: C 33.0, H 4.99, S 35.2, Zn 18.0.
FT-IR (cm−1): 2952 (w), 2868 (w), 1463 (w). 1189 (s), 1129 (m), 1040 (s), 939.3 (w), 736.3 (w), 665.1 (w).
Synthesis of bis(O-ethylxanthato)tin(II)
[Sn(S2COEt)2] was prepared by a procedure modified from that described in the literature [53, 56]. An aqueous solution of potassium ethylxanthate (10.0 g, 0.062 mol) was added to a solution of tin(II) chloride (5.90 g, 0.031 mol) in distilled water (100 ml) and stirred for a further 30 min. The yellow precipitate produced was collected by vacuum filtration, washed with water (3 × 50 ml) and finally dried in a vacuum oven at room temperature for 2 h to give [Sn(S2COEt)2] (7.20 g, 0.020 mol, 64% yield). MPt: 46–49 °C.
Calc. for C6H10O2S4Sn (%): C 20.0, H 2.79, S 35.5, Sn 32.9; found: C 19.7, H 2.74, S 35.5, Sn 32.2.
FT-IR (cm−1): 2986 (w), 2930 (w), 1457 (w), 1355 (w), 1196 (s), 1108 (s), 1021 (s), 852.0 (w), 801.3 (w), 563.4 (w).
Synthesis of (O-ethylxanthato)copper(I) triphenylphosphine
A solution of potassium ethylxanthate (0.641 g, 0.0040 mol) in chloroform (40 ml) was added to a solution of triphenylphosphine (2.09 g, 0.008 mol) and CuCl (0.40 g, 0.0040 mol) in the same amount of chloroform. A white precipitate was obtained after continuous stirring for 1 h at room temperature. The solution was filtered to obtain a clear yellow solution. Cooling the yellow solution to −20 °C gave yellow crystals of O-ethylxanthato copper(I) triphenylphosphine (2.40 g, 0.0033 mol, 85% yield). MPt: 185–191 °C.
Calc. for C39H35CuOP2S2 (%): C 66.1, H 4.97, S 9.02, P 8.74, Cu 8.96; found: C 65.7, H 5.08, S 8.77, P 8.44, Cu 8.74.
FT-IR (cm−1): 3048 (w), 2992 (w), 1478 (m) 1433 (m), 1290 (s), 1142 (m), 1041 (m), 1009 (s), 849.5 (s), 740.8 (m), 617.7 (s), 559.2 (s).
Additional precursors that were not used to make thin films are described in ESI.
Preparation of thin films
Glass slides were cut to 20 mm × 15 mm, cleaned by sonication in acetone and water and allowed to dry. Coating solutions were prepared by dissolving [(Ph3P)2CuS2COEt] (0.82 mmol), [Zn(S2COnBu)2] (0.41 mmol) and [Sn(S2COEt)2] (0.41 mmol) in THF (6 ml). A clear yellow solution was obtained. For each sample 2 ml of solution was coated onto the glass slide by spin coating at 700 rpm for 120 s and allowed to dry. The resulting films were then annealed in an N2 atmosphere with a heating ramp ~3 °C min−1 and held at the target temperature for 120 min; after this time had elapsed, the furnace was turned off and the tube allowed to cool to room temperature. The films were kept in the N2 atmosphere until they had cooled to room temperature.
Electrical measurements
The electrical properties of the thin films were investigated using the van der Pauw method [54]; contacts to the 7.5 × 7.5 mm CZTS thin films were obtained using pure In probes. A magnetic field of 0.088T strength was applied during the Hall measurements. The values of resistance between each In contact pairs were homogeneous, and the four individual Hall voltages were close to each in value giving a statistically meaningful average. The film resistivity was calculated from the Hall voltage and sheet resistance.
Results and discussion
A range of copper, zinc and tin ethyl- and n-butylxanthate complexes were synthesized by the reaction of the appropriate metal chloride with the relevant potassium xanthate. The suitability of these complexes for melt reactions was assessed through measurements of their thermal stability.
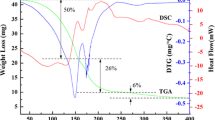
Thermogravimetric analysis (TGA, Fig. 1) demonstrates the decomposition range of the Sn, Zn and Cu xanthates in a nitrogen atmosphere. An optimum mixture of precursors for melt reactions is one in which the precursors all decompose at similar reaction temperatures.
[Sn(S2COEt)2] decomposes cleanly in TGA at 100–145 °C, whereas [Zn(S2COnBu)2] decomposes via a two-step process between 110–220 and 250–350 °C. The decomposition of [(Ph3P)2CuS2COEt] shows a mass loss starting at about 140 °C and finishing at 260 °C. The TGA curves indicate that SnS and CuS are formed cleanly, while ZnS contains impurities up to 350 °C. Sn(S2COEt)4 and (Ph3P)2(Cu(S2COnBu)2 have hence been discounted as viable precursors owing to their decomposition temperatures that differ from other precursors. The ethyl xanthate of zinc [Zn(S2COEt)2] is much more hydroscopic than its n-butyl cousin. Therefore, [(Ph3P)2CuS2COEt], [Zn(S2COnBu)2] and [Sn(S2COEt)2] were chosen to deposit CZTS films. Additionally, these complexes are readily soluble in many common organic solvents.
CZTS thin films were prepared using a solution of [(Ph3P)2CuS2COEt] (0.82 mmol), [Zn(S2COnBu)2] (0.41 mmol) and [Sn(S2COEt)2] (0.41 mmol) dissolved in THF (6 ml) and spin coated onto a glass slide. The resulting films were then heated in an N2 atmosphere at the desired temperature (between 200 and 475 °C) for 120 min. The CZTS films were gray for all of the heating temperatures. Scanning electron microscopy (SEM) images (Fig. 2) reveal the surface morphology of the films to be predominantly granular for the films prepared at temperatures >300 °C, with particles in the region of 0.25 μm in diameter. The films prepared at lower temperature have a more flake-like consistency and contain much smaller particles. Side-on SEM images (ESI Figure S5) show that the films are 1.85–2.0 μm in thickness.
Energy-dispersive X-ray spectroscopy (EDX) analysis shows the presence of copper, zinc, tin and sulfur in the films. It is important to determine the Zn/Sn and Cu/(Zn + Sn) ratios within the films as slight changes in these values can lead to significant changes in structural/electronic properties [57]. The EDX measured compositions of the as-prepared films are shown in ESI Table S1.
The Cu/(Zn + Sn) ratio is in the range of 0.8–1.0 (ESI Table S1). The films heated at 225 °C gave Cu/(Zn + Sn) > 1, whereas the other films heated at high temperatures are copper deficient (ESI Table S1), which may reflect the volatility of the precursors. The slight copper deficiency is a promising result as solar cells made from Cu-poor films perform substantially better than those made from stoichiometric Cu2ZnSnS4 [18].
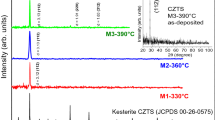
The p-XRD patterns for the samples prepared at temperatures between 200 and 475 °C are shown in Fig. 3. It is clear that the peaks sharpen with increased heating temperatures, with the width of the (002)/(112) reflection decreasing, which indicates improved crystallinity or increased crystalline size.
a p-XRD patterns and b Raman scattering spectra of CZTS thin films annealed in an N2 atmosphere at the desired temperature (200–475 °C) for 120 min. Chalcocite (Cu2−xS) is identifiable within the p-XRD for temperatures <300 °C: low-intensity peaks for the (110) plane (2θ = 46.7°) and (103) plane (2θ = 48.8°)
At temperatures ≥375 °C simpler diffraction patterns are seen than at lower temperatures. Only KS-CZTS was observed—this grows with a strongly preferred (112) orientation and matches well with the tetragonal CZTS standard (JCPDS No. 04-017-3032) which has a space group Ī4. The lattice parameters of a = 5.431 Å, b = 5.431 Å and c = 10.844 Å (ESI Tables S4, S5 and S6) match well with the literature values and indicate that the material is tetragonal and not cubic [58].
The films prepared at 200–250 °C consist of a relatively poorly crystalline hexagonal CZTS (WZ-CZTS, ESI Tables S2 and S6). The wurtzitic form of CZTS has previously been seen for nanoparticles and thin films [37, 59]. The diffraction patterns of the hexagonal and orthorhombic phases are very similar. However, in orthorhombic CZTS the low angle peaks (100) and (101) in Fig. 3a appear as ‘doublets’, representing the (210) + (020) and (211) + (021) lattice planes, respectively [60]. This splitting is not observed in our p-XRD pattern, nor is it seen in the selected area electron diffraction (SAED, Fig. 5a and ESI Table S2); indeed, the patterns from TEM are very sharp and clear. Therefore, we conclude that the low-temperature films result in the WZ-CZTS phase. Some contamination with binary or ternary phases may occur, as can be observed in the films prepared at <300 °C in which chalcocite (Cu2−xS) is identifiable within the diffraction patterns.
The Raman spectra for the CZTS films give a good indication of the phase formed (Fig. 3b). Kesteritic CZTS has a relatively narrow and dominant Raman shift at 338 cm−1 from the A1 mode, with further peaks at 288 (A mode), 358 (B mode) and 372 cm−1 (B mode) [61, 62]. This is seen in the films heated at higher temperatures (>400 °C). For the samples prepared at lower temperature (<300 °C), the major peak has a shift of 327 cm−1 (ESI Figure S3). This corresponds to the WZ-CZTS A mode [63]. This indicates that the low-temperature films are wurtzite CZTS and there is a phase transition to kesterite at higher temperatures, in agreement with the p-XRD data. We note that there is also a peak in the Raman spectra for the 200 °C film at 468 cm−1, which corresponds to Cu2S.
Increasing in temperature leads to a gradual narrowing of the bands, and Raman bands appear with frequencies of about 331, 333 and 337 cm−1. Films heated at 475 °C showed a higher intensity band at 337 cm−1 (ESI Figure S4), which is indicative of KS–CZTS [64].
Figure 4 shows the dependence of the shift of the dominant Raman peak on the heating temperatures. The WZ-CZTS films have a major peak at 326–327 cm−1, and this can clearly be seen for the samples prepared at <350 °C. As the annealing temperature increases, the peak moves toward 336 cm−1, representing the presence of kesterite. This is demonstrated by the rapid increase in Raman shift seen from 375 to 475 °C. The p-XRD data show that the films prepared at 400–450 °C are KS-CZTS too, and the downshifted peak is due to disorder in Cu and Zn atoms in the sublattice. This is because the Cu and Zn atoms have a similar size and the difference between binding energy of the cations in the sublattice is small [65, 66].
The position of the dominant Raman peak at 326–336 cm−1 in Fig. 3b and its relationship to the annealing temperature of the films
Samples suitable for transmission electron microscope (TEM) imaging were prepared by ultrasonication of the CZTS film (produced at 225, 350 and 450 °C, Fig. 5). Closer inspection of the CZTS produced at 225 °C found that two distinct kinds of crystalline materials were present: a flake-like material and short nanorods with lattices fringes present (Fig. 5a). Both sets of crystals were typically <40 and 20 nm in diameter, respectively. We were able to observe patches on the TEM grid that consisted mainly of either phase. The selected area electron diffraction (SAED) patterns found distinct rings—indicative of polycrystalline materials, with these rings consistent with the identification of hexagonal (flake-like) and cubic (rod-like) phases of CZTS. This SAED, inset to Fig. 5a, shows an interplanar spacing of 0.27 and 0.19 nm, which correspond to the (200) and (220) planes of cubic CZTS (ESI Table S3). The interplanar spacing of 0.33 and 0.32 nm relates to the (100) and (002) planes of hexagonal CZTS (ESI Table S2). Two phases (hexagonal and cubic) can be seen in the p-XRD pattern of the film heated at 225 °C (Fig. 3a) due to the equivalence of the (002) planes of the hexagonal structure with the (111) planes of the cubic structure.
TEM images of Cu2ZnSnS4 nanocrystals. a A CZTS film heated at 225 °C. The insets show selected area electron diffraction (SAED) images, gray box for cubic CZTS and white box for hexagonal CZTS. b A film heated at 350 °C. The SAED pattern can be indexed to tetragonal CZTS. c A film heated at 450 °C, the inset SAED pattern is also indexed to tetragonal CZTS
Exfoliation of CZTS films produced at 350 °C showed a crystalline material (<20 nm); SAED supported the formation of only the tetragonal phase of kesterite CZTS (Fig. 5b). The interplanar spacing of 0.31, 0.19 and 0.16 nm corresponds to the (112), {(204)(220)} and {(312)(116)} planes of kesterite CZTS (ESI Table S4). Likewise, for the film heated to 450 °C, we only observed crystalline particles <20 nm, and for which the SAED could also be indexed to tetragonal kesterite CZTS (Fig. 5c, ESI Table S5).
The electrical parameters of the WZ-CZTS film prepared at 225 °C (ESI S6) and the phase pure KS-CZTS samples annealed at 375 °C (ESI S7) and 450 °C (ESI S8) were obtained using the van der Pauw four-probe configuration in Hall effect measurements at room temperature. All samples exhibited p-type conductivity, a desirable requirement for the fabrication of heterojunction solar cells.
The resistivity (ρ), Hall mobility (μ), carrier concentration (p) and Hall coefficient (R H) are shown in Table 1. The resistivity (ρ) decreased from 27.1 Ω cm to around 1.23 Ω cm for the transition from WZ-CZTS to KS-CZTS. In addition, we found that the Hall coefficient (R H) decreased from 2.36 × 10+3 cm3 C−1 for WZ-CZTS films to 13.7 cm3 C−1 for KS-CZTS °C. We have determined that for the two phases, the Hall mobility (μ) is 87.1 and 11.1 cm2 V−1 s−1, respectively. The carrier concentration values were 2.65 × 10+15 and 4.55 × 10+17 cm−3, the latter of which is an excellent value for use in practical devices.
TEM images of the film prepared at 225 °C indicate that the film exhibited hexagonal CZTS as well as cubic (Fig. 5a). This confirms the presence of impurities in the predominantly wurtzite phase CZTS. McGill [67] and Hall [68] previously showed that as the impurity concentration increases, the mobility decreases. This behavior has also been seen for Si [69] and GaAs [70]. This model can be applied to the CZTS system, as moving from 225 to 375/450 °C we see a substantial decrease in mobility.
Conclusions
Thermogravimetric analysis was used to investigate the decomposition of a series of O-n-butyl and O-ethylxanthate complexes of copper, tin and zinc. [(Ph3P)2CuS2COEt], [Zn(S2COnBu)2] and [Sn(S2COEt)2] were found to have compatible decomposition temperatures and were used for the formation of Cu2ZnSnS4 (CZTS) by spin coating followed by heating under N2. The p-XRD patterns of CZTS thin films were obtained after heating at temperatures between 200 and 475 °C and revealed a temperature dependence of the CZTS phase formed. Higher temperatures give the normal tetragonal phase CZTS, while low temperatures are mixed hexagonal and cubic phases. EDX measurements show that the Cu/(Zn + Sn) ratio was between 1 and 0.64. The measured resistivity, carrier concentration, mobility and Hall coefficient of films heated at 225, 375 and 450 °C, indicated fairly homogenous films. In this work the wurtzite CZTS has a much higher resistivity and mobility, but a much lower charge carrier density.
References
Woo K, Kim Y, Moon J (2012) A non-toxic, solution-processed, earth abundant absorbing layer for thin-film solar cells. Energy Environ Sci 5:5340–5345. doi:10.1039/c1ee02314d
Katagiri H, Jimbo K, Maw WS, Oishi K, Yamazaki M, Araki H et al (2009) Development of CZTS-based thin film solar cells. Thin Solid Films 517:2455–2460. doi:10.1016/j.tsf.2008.11.002
Matthews PD, McNaughter PD, Lewis DJ, O’Brien P (2017) Shining a light on transition metal chalcogenides for sustainable photovoltaics. Chem Sci 8:4177–4187. doi:10.1039/C7SC00642J
Shin B, Gunawan O, Zhu Y, Bojarczuk NA, Chey SJ, Guha S (2013) Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog Photovolt Res Appl 21:72–76. doi:10.1002/pip.1174
Saha SK, Guchhait A, Pal AJ (2012) Cu2ZnSnS4 (CZTS) nanoparticle based nontoxic and earth-abundant hybrid pn-junction solar cells. Phys Chem Chem Phys 14:8090–8096. doi:10.1039/c2cp41062a
Scragg JJ, Dale PJ, Peter LM, Zoppi G, Forbes I (2008) New routes to sustainable photovoltaics: evaluation of Cu2ZnSnS4 as an alternative absorber material. Phys Status Solidi 245:1772–1778. doi:10.1002/pssb.200879539
Jimbo K, Kimura R, Kamimura T, Yamada S, Maw WS, Araki H et al (2007) Cu2ZnSnS4-type thin film solar cells using abundant materials. Thin Solid Films 515:5997–5999. doi:10.1016/j.tsf.2006.12.103
Ito K, Nakazawa T (1988) Electrical and optical properties of stannite-type quaternary semiconductor thin films. Jpn J Appl Phys 27:2094–2097. doi:10.1143/JJAP.27.2094
Katagiri H, Sasaguchi N, Hando S, Hoshino S, Ohashi J, Yokota T (1997) Preparation and evaluation of Cu2ZnSnS4 thin films by sulfurization of E-B evaporated precursors. Sol Energy Mater Sol Cells 49:407–414. doi:10.1016/S0927-0248(97)00119-0
Shin SW, Pawar SM, Park CY, Yun JH, Moon JH, Kim JH et al (2011) Studies on Cu2ZnSnS4 (CZTS) absorber layer using different stacking orders in precursor thin films. Sol Energy Mater Sol Cells 95:3202–3206. doi:10.1016/j.solmat.2011.07.005
Suryawanshi MP, Agawane GL, Bhosale SM, Shin SW, Patil PS, Kim JH et al (2013) CZTS based thin film solar cells: a status review. Mater Technol 28:98–109. doi:10.1179/1753555712y.0000000038
Kumar M, Dubey A, Adhikari N, Venkatesan S, Qiao Q (2015) Strategic review of secondary phases, defects and defect-complexes in kesterite CZTS–Se solar cells. Energy Environ Sci 8:3134–3159. doi:10.1039/C5EE02153G
Ramasamy K, Malik MA, O’Brien P (2011) The chemical vapor deposition of Cu2ZnSnS4 thin films. Chem Sci 2:1170–1172. doi:10.1039/c0sc00538j
Schurr R, Holzing A, Jost S, Hock R, Voss T, Schulze J et al (2009) The crystallisation of Cu2ZnSnS4 thin film solar cell absorbers from co-electroplated Cu-Zn-Sn precursors. Thin Solid Films 517:2465–2468. doi:10.1016/j.tsf.2008.11.019
Wangperawong A, King JS, Herron SM, Tran BP, Pangan-Okimoto K, Bent SF (2011) Aqueous bath process for deposition of Cu2ZnSnS4 photovoltaic absorbers. Thin Solid Films 519:2488–2492. doi:10.1016/j.tsf.2010.11.040
Su Z, Yan C, Sun K, Han Z, Liu F, Liu J et al (2012) Preparation of Cu2ZnSnS4 thin films by sulfurizing stacked precursor thin films via successive ionic layer adsorption and reaction method. Appl Surf Sci 258:7678–7682. doi:10.1016/j.apsusc.2012.04.120
Chen LJ, Chuang YJ (2013) Quaternary semiconductor derived and formation mechanism by non-vacuum route from solvothermal nanostructures for high-performance application. Mater Lett 91:372–375. doi:10.1016/j.matlet.2012.10.037
Johnson MC, Wrasman C, Zhang X, Manno M, Leighton C, Aydil ES (2015) Self-regulation of Cu/Sn ratio in the synthesis of Cu2ZnSnS4 films. Chem Mater 27:2507–2514. doi:10.1021/acs.chemmater.5b00108
Feng Y, Yu B, Cheng G, Lau T, Li Z, Yin L et al (2015) Searching for a fabrication route of efficient Cu2ZnSnS4 solar cells by post-sulfuration of co-sputtered Sn-enriched precursors. J Mater Chem C 3:9650–9656. doi:10.1039/C5TC02486B
Scragg JJ, Ericson T, Kubart T, Edo M, Platzer-Bjorkman C (2011) Chemical insights into the instability of Cu2ZnSnS4 Films during annealing. Chem Mater 23:4625–4633. doi:10.1021/cm202379s
Yu K, Carter EA (2015) A strategy to stabilize kesterite CZTS for high-performance solar cells. Chem Mater 27:2920–2927. doi:10.1021/acs.chemmater.5b00172
Yu K, Carter EA (2016) Determining and controlling the stoichiometry of Cu2ZnSnS4 photovoltaics: the physics and its implications. Chem Mater 28:4415–4420. doi:10.1021/acs.chemmater.6b01612
Scragg JJ, Kubart T, Wätjen JT, Ericson T, Linnarsson MK, Platzer-Björkman C (2013) Effects of back contact instability on Cu2ZnSnS4 devices and processes. Chem Mater 25:3162–3171. doi:10.1021/cm4015223
Jiang F, Gunawan T, Harada Y, Kuang T, Minegishi K Domen et al (2015) Pt/In2S3/CdS/Cu2ZnSnS4 thin film as an efficient and stable photocathode for water reduction under sunlight radiation. J Am Chem Soc 137:13691–13697. doi:10.1021/jacs.5b09015
Chang J, Waclawik ER (2014) Colloidal semiconductor nanocrystals: controlled synthesis and surface chemistry in organic media. RSC Adv 4:23505–23527. doi:10.1039/c4ra02684e
Chen S, Gong XG, Walsh A, Wei SH (2009) Crystal and electronic band structure of Cu2ZnSnX4 (X = S and Se) photovoltaic absorbers: first-principles insights. Appl Phys Lett 94:25–27. doi:10.1063/1.3074499
Paier J, Asahi R, Nagoya A, Kresse G (2009) Cu2ZnSnS4 as a potential photovoltaic material: a hybrid Hartree-Fock density functional theory study. Phys Rev B 79:115126. doi:10.1103/PhysRevB.79.115126
Khare A, Himmetoglu B, Johnson M, Norris DJ, Cococcioni M, Aydil ES (2012) Calculation of the lattice dynamics and Raman spectra of copper zinc tin chalcogenides and comparison to experiments. J Appl Phys 111:83707. doi:10.1063/1.4704191
Bernard JE, Ferreira LG, Wei S-H, Zunger A (1988) Ordering of isovalent intersemiconductor alloys. Phys Rev B 38:6338–6341. doi:10.1017/CBO9781107415324.004
Magri R, Wei S-H, Zunger A (1990) Ground-state structures and the random-state energy of the Madelung lattice. Phys Rev B 42:11388–11391
Chen S, Walsh A, Luo Y, Yang JH, Gong XG, Wei SH (2010) Wurtzite-derived polytypes of kesterite and stannite quaternary chalcogenide semiconductors. Phys Rev B 82:195203. doi:10.1103/PhysRevB.82.195203
Shibuya T, Goto Y, Kamihara Y, Matoba M, Yasuoka K, Burton LA et al (2014) From kesterite to stannite photovoltaics: stability and band gaps of the Cu2(Zn, Fe)SnS4 alloy. Appl Phys Lett 104:21912. doi:10.1063/1.4862030
Jackson AJ, Walsh A (2014) Ab initio thermodynamic model of Cu2ZnSnS4. J Mater Chem A 2:7829–7836. doi:10.1039/c4ta00892h
Ramasamy K, Malik MA, O’Brien P (2012) Routes to copper zinc tin sulfide Cu2ZnSnS4 a potential material for solar cells. Chem Commun 48:5703–5714. doi:10.1039/c2cc30792h
Schorr S, Gonzalez-Aviles G (2009) In-situ investigation of the structural phase transition in kesterite. Phys Status Solidi 206:1054–1058. doi:10.1002/pssa.200881214
Ahmad R, Brandl M, Distaso M, Herre P, Spiecker E, Hock R et al (2015) A comprehensive study on the mechanism behind formation and depletion of Cu2ZnSnS4 (CZTS) phases. CrystEngComm 17:6972–6984. doi:10.1039/C5CE00661A
Cattley CA, Cheng C, Fairclough SM, Droessler LM, Young NP, Warner JH et al (2013) Low temperature phase selective synthesis of Cu2ZnSnS4 quantum dots. Chem Commun 49:3745–3747. doi:10.1039/c3cc39042j
Nakayama N, Ito K (1996) Sprayed films of stannite Cu2ZnSnS4. Appl Surf Sci 92:171–175. doi:10.1016/0169-4332(95)00225-1
Botti S, Kammerlander D, Marques MAL (2011) Band structures of Cu2ZnSnS4 and Cu2ZnSnSe4 from many-body methods. Appl Phys Lett 98:241915. doi:10.1063/1.3600060
Lund EA, Scarpulla MA (2013) Modeling Cu2ZnSnS4 (CZTS) solar cells with kesterite and stannite phase variation. Proc SPIE 8620:862015. doi:10.1117/12.2012401
Kevin P, Malik MA, O’Brien P (2015) The controlled deposition of Cu2(ZnyFe1−y)SnS4, Cu2(ZnyFe1−y)SnSe4 and Cu2(ZnyFe1−y)Sn(SxSe1−x)4 thin films by AACVD: potential solar cell materials based on earth abundant elements. J Mater Chem C 3:5733–5741. doi:10.1039/C5TC00867K
Kevin P, Malik MA, O’Brien P (2015) The AACVD of Cu2FeSn(SxSe1−x)4: potential environmentally benign solar cell materials. New J Chem 39:7046–7053. doi:10.1039/C5NJ01198A
Kevin P, Malik MA, Mcadams S, O’Brien P (2015) Synthesis of nanoparticulate alloys of the composition Cu2Zn1−xFexSnS4: structural, Optical, and Magnetic Properties. J Am Chem Soc 137:15086–15089. doi:10.1021/jacs.5b10281
Lewis EA, McNaughter PD, Yin Z, Chen Y, Brent JR, Saah SA et al (2015) In situ synthesis of PbS nanocrystals in polymer thin films from lead(II) xanthate and dithiocarbamate complexes: evidence for size and morphology control. Chem Mater 27:2127–2136. doi:10.1021/cm504765z
Lewis EA, Haigh S, O’Brien P (2014) The synthesis of metallic and semiconducting nanoparticles from reactive melts of precursors. J Mater Chem A 2:570–580. doi:10.1039/C3TA12900D
Matthews PD, Akhtar M, Malik MA, Revaprasadu N, O’Brien P (2016) Synthetic routes to iron chalcogenide nanoparticles and thin films. Dalton Trans 45:18803–18812. doi:10.1039/C6DT03486A
Lewis DJ, Tedstone AA, Zhong XL, Lewis EA, Rooney A, Savjani N et al (2015) Thin films of molybdenum disulfide doped with chromium by aerosol-assisted chemical vapor deposition (AACVD). Chem Mater 27:1367–1374. doi:10.1021/cm504532w
Savjani N, Brent JR, O’Brien P (2015) AACVD of molybdenum sulfide and oxide thin films from molybdenum(V)-based single-source precursors. Chem Vap Depos 21:71–77. doi:10.1002/cvde.201407135
Savjani N, Lewis EA, Bissett MA, Brent JR, Dryfe RAW, Haigh SJ et al (2016) Synthesis of lateral size-controlled monolayer 1H-MoS2@Oleylamine as supercapacitor electrodes. Chem Mater 28:657–664. doi:10.1021/acs.chemmater.5b04476
Al-Shakban M, Xie Z, Savjani N, Malik MA, O’Brien P (2016) A facile method for the production of SnS thin films from melt reactions. J Mater Sci 51:6166–6172. doi:10.1007/s10853-016-9906-7
Ramasamy K, Malik MA, Revaprasadu N, O’Brien P (2013) Routes to nanostructured inorganic materials with potential for solar energy applications. Chem Mater 25:3551–3569. doi:10.1021/cm401366q
Alam N, Hill MS, Kociok-Köhn G, Zeller M, Mazhar M, Molloy KC (2008) Pyridine adducts of nickel(II) xanthates as single-source precursors for the aerosol-assisted chemical vapor deposition of nickel sulfide. Chem Mater 20:6157–6162. doi:10.1021/cm801330v
Kociok-Köhn G, Molloy KC, Sudlow AL (2014) Molecular routes to Cu2ZnSnS4: a comparison of approaches to bulk and thin-film materials. Can J Chem 92:514–524. doi:10.1139/cjc-2013-0497
van der Pauw LJ (1958) A method of measuring specific resistivity and hall effect of discs of arbitrary shape. Philips Res Rep 13:1–9. doi:10.1017/CBO9781107415324.004
Mohamed AA, Kani I, Ramirez AO, Fackler JP (2004) Synthesis, characterization, and luminescent properties of Dinuclear Gold(I) Xanthate complexes: X-ray structure of [Au2(nBu-xanthate)2]. Inorg Chem 43:3833–3839. doi:10.1021/ic0349858
Raston CL, Tennant PR, White AH, Winter G (1978) Reactions of tin(II) and tin(IV) xanthates: crystal structure of Tetrakis (O-ethylxanthato) tin(IV). Aust J Chem 31:1493–1500. doi:10.1071/CH9781493
Zhou S, Tan R, Jiang X, Shen X, Xu W, Song W (2013) Growth of CZTS thin films by sulfurization of sputtered single-layered Cu-Zn-Sn metallic precursors from an alloy target. J Mater Sci Mater Electron 24:4958–4963. doi:10.1007/s10854-013-1507-5
Bonazzi P, Bindi L, Bernardini GP, Menchetti S (2003) A model for the mechanism of incorporation of Cu, Fe, and Zn in the Stannite–Kesterite series, Cu2FeSnS4–Cu2ZnSnS4. Can Mineralogist 41:639–647. doi:10.2113/gscanmin.41.3.639
Mainz R, Singh A, Levcenko S, Klaus M, Genzel C, Ryan KM et al (2014) Phase-transition-driven growth of compound semiconductor crystals from ordered metastable nanorods. Nat Commun 5:3133. doi:10.1038/ncomms4133
Jiang H, Dai P, Feng Z, Fan W, Zhan J (2012) Phase selective synthesis of metastable orthorhombic Cu2ZnSnS4. J Mater Chem 22:7502–7506. doi:10.1039/c2jm16870g
Cheng A-J, Manno M, Khare A, Leighton C, Campbell SA, Aydil ES (2011) Imaging and phase identification of Cu2ZnSnS4 thin films using confocal Raman spectroscopy. J Vac Sci Technol A 29:51203. doi:10.1116/1.3625249
Dimitrievska M, Fairbrother A, Fontane X, Jawhari T, Izquierdo-Roca V, Saucedo E et al (2014) Multiwavelength excitation Raman scattering study of polycrystalline kesterite Cu2ZnSnS4 thin films. Appl Phys Lett 104:21901. doi:10.1063/1.4861593
Tan JMR, Lee YH, Pedireddy S, Baikie T, Ling XY, Wong LH (2014) Understanding the synthetic pathway of single phase quarternary semiconductor using surface-enhanced Raman scattering: a case of Wurtzite Cu2ZnSnS4 nanoparticles. J Am Chem Soc 136:6684–6692. doi:10.1021/ja501786s
Himmrich M, Haeuseler H (1991) Far infrared studies on stannite and wurtzstannite type compounds. Spectrochim Acta 47A:933–942. doi:10.1016/0584-8539(91)80283-O
Valakh MY, Kolomys OF, Ponomaryov SS, Yukhymchuk VO, Babichuk IS, Izquierdo-Roca V et al (2013) Raman scattering and disorder effect in Cu2ZnSnS4. Phys Status Solidi Rapid Res Lett 7:258–261. doi:10.1002/pssr.201307073
Walsh A, Chen S, Wei SH, Gong XG (2012) Kesterite thin-film solar cells: advances in materials modelling of Cu2ZnSnS4. Adv Energy Mater 2:400–409. doi:10.1002/aenm.201100630
McGill TC, Baron R (1975) Neutral impurity scattering in semiconductors. Phys Rev B 11:5208–5210
Hall GL (1962) Ionized impurity scattering in semiconductors. J Phys Chem Solids 23:1147–1151. doi:10.1103/PhysRevB.20.3254
Kosina H, Kaiblinger-Grujin G (1998) Ionized-impurity scattering of majority electrons in silicon. Solid State Electron 42:331–338. doi:10.1016/S0038-1101(97)00199-8
Wolfe CM, Stillman GE, Dimmock JO (1970) Ionized impurity density in n-type GaAs. J Appl Phys 41:504–507. doi:10.1063/1.1658704
Acknowledgements
The authors would like to acknowledge the Iraqi Culture Attaché in London for financial support (M.A.S.) and the EPSRC (Doctoral Prize for P.D.M., EP/M507969/1). N.S. thanks Carl and Anne Parker for the fellowship that funded this work. Some of the equipment used in this study were provided by the Engineering and Physical Sciences Research Council (Core Capability in Chemistry, EPSRC Grant Number EP/K039547/1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Shakban, M., Matthews, P.D., Savjani, N. et al. The synthesis and characterization of Cu2ZnSnS4 thin films from melt reactions using xanthate precursors. J Mater Sci 52, 12761–12771 (2017). https://doi.org/10.1007/s10853-017-1367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1367-0