Abstract
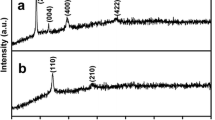
Cu3Mo2O9 nanorods (NRs) have been prepared via a facile hydrothermal method with subsequent heat treatment. The sample is characterized by the XRD, SEM, TEM, and XPS techniques. The results indicate that Cu3Mo2O9 NRs crystallize in the orthorhombic space group Pnma and have the diameters at around 100 nm. As the anode material for lithium-ion batteries, Cu3Mo2O9 NRs can deliver an initial discharge capacity of 1209 mAh/g at a current density of 300 mA/g in the potential window of 0.005–3 V, and a reversible capacity of 440 mAh/g can be maintained after 400 cycles. Furthermore, Cu3Mo2O9 NRs still have the capacity of 437 mAh/g even at 1 A/g. The improved electrochemical performance of Cu3Mo2O9 can be attributed to the effect of enhanced contact area and shortened Li+ ions’ transport distance between the Cu3Mo2O9 NRs and the electrolyte.









Similar content being viewed by others
References
Ghadbeigi L, Harada JK, Lettiere BR, Sparks TD (2015) Performance and resource considerations of Li-ion battery electrode materials. Energy Environ Sci 8:1640–1650
Dunn JB, Gaines L, Kelly JC, James C, Gallagher KG (2015) The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ Sci 8:158–168
Yan Y, Gu P, Zheng S, Zheng M, Pang H, Xue H (2016) Facile synthesis of an accordion-like Ni-MOF superstructure for high-performance flexible supercapacitors. J Mater Chem A 4:19078–19085
Yabuuchi N, Kubota K, Dahbi M, Komaba S (2014) Research development on sodium-ion batteries. Chem Rev 114:11636–11682
Li B, Gu P, Feng Y, Zhang G, Huang K, Xue H, Pang H (2017) Ultrathin nickel–cobalt phosphate 2D nanosheets for electrochemical energy storage under aqueous/solid–state electrolyte. Adv Funct Mater 27:1605784
Goodenough JB, Park KS (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135:1167–1176
Ni L, Wu Z, Zhao G, Sun C, Zhou C, Gong X, Diao G (2017) Core–shell structure and interaction mechanism of γ-MnO2 coated sulfur for improved lithium–sulfur batteries. Small 13:1603466
Zhang J, Gu P, Xu J, Xue H, Pang H (2016) High performance of electrochemical lithium storage batteries: ZnO-based nanomaterials for lithium-ion and lithium–sulfur batteries. Nanoscale 8:18578–18595
Kang WP, Tang YB, Li WY, Yang X, Xue HT, Yang QD, Lee CS (2015) High interfacial storage capability of porous NiMn2O4/C hierarchical tremella-like nanostructures as the lithium ion battery anode. Nanoscale 7:225–231
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Chen M, Li W, Shen X, Diao G (2014) Fabrication of core–shell α-Fe2O3@Li4Ti5O12 composite and its application in the lithium ion batteries. ACS Appl Mater Interfaces 6:4514–4523
Ju Z, Zhang E, Zhao Y, Xing Z, Zhuang Q, Qiang Y, Qian Y (2015) One-pot hydrothermal synthesis of FeMoO4 nanocubes as an anode material for lithium-ion batteries with excellent electrochemical performance. Small 11:4753–4761
Yao J, Gong Y, Yang S, Xiao P, Zhang Y, Keyshar K, Ye G, Ozden S, Vajtai R, Ajayan PM (2014) CoMoO4 nanoparticles anchored on reduced graphene oxide nanocomposites as anodes for long-life lithium-ion batteries. ACS Appl Mater Interfaces 6:20414–20422
Zhang Z, Li W, Ng TW, Kang W, Lee CS, Zhang W (2015) Iron (ii) molybdate (FeMoO4) nanorods as a high-performance anode for lithium ion batteries: structural and chemical evolution upon cycling. J Mater Chem A 3:20527–20534
Xiao W, Chen JS, Li CM, Xu R, Lou XW (2010) Synthesis, characterization, and lithium storage capability of AMoO4 (A = Ni, Co) nanorods. Chem Mater 22:746–754
Xia J, Song LX, Liu W, Teng Y, Wang QS, Zhao L, Ruan MM (2015) Highly monodisperse Cu3Mo2O9 micropompons with excellent performance in photocatalysis, photocurrent response and lithium storage. RSC Adv 5:12015–12024
Xia J, Song LX, Liu W, Teng Y, Wang QS, Zhao L, Ruan MM (2015) Construction of Cu3Mo2O9 nanoplates with excellent lithium storage properties based on a pH-dependent dimensional change. Dalton Trans 44:13450–13454
Peng G, Wu S, Ellis JE, Xu X, Xu G, Yu C, Star A (2016) Single-walled carbon nanotubes templated CuO networks for gas sensing. J Mater Chem C 4:6575–6580
Pramanik P (1999) A novel chemical route for the preparation of nanosized oxides, phosphates, vanadates, molybdates and tungstates using polymer precursors. Bull Mater Sci 22:335–339
Cherian CT, Reddy MV, Haur SC, Chowdari BVR (2013) Interconnected network of CoMoO4 submicrometer particles as high capacity anode material for lithium ion batteries. ACS Appl Mater Interfaces 5:918–923
Ma FX, Wang PP, Xu CY, Yu J, Fang HT, Zhen L (2014) Synthesis of self-stacked CuFe2O4–Fe2O3 porous nanosheets as a high performance Li-ion battery anode. J Mater Chem A 2:19330–19337
Zhang X, Liu HH, Petnikota S, Ramakrishna S, Fan HJ (2014) Electrospun Fe2O3-carbon composite nanofibers as durable anode materials for lithium ion batteries. J Mater Chem A 2:10835–10841
Xing Z, Ju ZC, Yang J, Xu HY, Qian YT (2013) One-step solid state reaction to selectively fabricate cubic and tetragonal CuFe2 O4 anode material for high power lithium ion batteries. Electrochim Acta 102:51–57
Banerjee A, Singh U, Aravindan V, Srinivasan M, Ogale S (2013) Synthesis of CuO nanostructures from Cu-based metal organic framework (MOF-199) for application as anode for Li-ion batteries. Nano Energy 2:1158–1163
Wang C, Wan W, Huang YH, Chen JT, Zhou HH, Zhang XX (2014) Hierarchical MoS2 nanosheet/active carbon fiber cloth as a binder-free and free-standing anode for lithium-ion batteries. Nanoscale 6:5351–5358
Ding SJ, Zhang DY, Chen JS, Lou XW (2012) Facile synthesis of hierarchical MoS2 microspheres composed of few-layered nanosheets and their lithium storage properties. Nanoscale 4:95–98
Meduri P, Clark E, Kim JH, Dayalan E, Sumanasekera GU, Sunkara MK (2012) MoO3–x nanowire arrays as stable and high-capacity anodes for lithium ion batteries. Nano Lett 12:1784–1788
Jin L, Qiu Y, Deng H, Li W, Li H, Yang S (2011) Hollow CuFe2O4 spheres encapsulated in carbon shells as an anode material for rechargeable lithium-ion batteries. Electrochim Acta 56:9127–9132
Zhou GM, Wang DW, Yin LC, Li N, Li F, Cheng HM (2012) Oxygen bridges between NiO nanosheets and graphene for improvement of lithium storage. ACS Nano 6:3214–3223
Ma Y, Zhang C, Ji G, Lee JY (2012) Nitrogen-doped carbon-encapsulation of Fe3O4 for increased reversibility in Li+ storage by the conversion reaction. J Mater Chem 22:7845–7850
Sun HT, Xin GQ, Hu T, Yu MP, Shao DL, Sun X, Lian J (2014) High-rate lithiation-induced reactivation of mesoporous hollow spheres for long-lived lithium-ion batteries. Nat Commun 5:4526
Sharma N, Shaju KM, Rao GVS, Chowdari BVR, Dong ZL, White TJ (2004) Carbon-coated nanophase CaMoO4 as anode material for Li ion batteries. Chem Mater 16:504–512
Xiao W, Chen JS, Li CM, Xu R, Lou XW (2009) Synthesis, characterization, and lithium storage capability of AMoO4 (A = Ni, Co) nanorods. Chem Mater 22:746–754
Guan BQ, Sun WW, Wang Y (2016) Carbon-coated MnMoO4 nanorod for high-performance lithium-ion batteries. Electrochim Acta 190:354–359
Wu R, Wang D, Rui X, Liu B, Zhou K, Law A, Yan Q, Wei J, Chen Z (2015) In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv Mater 27:3038–3044
Liu J, Wen Y, Wang Y, Aken PAV, Maier J, Yu Y (2014) Carbon-encapsulated pyrite as stable and earth-abundant high energy cathode material for rechargeable lithium batteries. Adv Mater 26:6025–6030
Acknowledgements
This study was funded by the financial support by the NSF of China (21673203), the Higher Education Science Foundation of Jiangsu Province (15KJB150031), Natural Science Foundation of Yangzhou (YZ2016122), Qing Lan Project and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, JC., Feng, F., Yang, SH. et al. Promising electrochemical performance of Cu3Mo2O9 nanorods for lithium-ion batteries. J Mater Sci 52, 12380–12389 (2017). https://doi.org/10.1007/s10853-017-1360-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1360-7