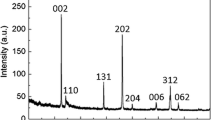
Abstract
The γ-LiFeO2 micro-cubes were synthesized using a simple solid-state method. Their electrochemical performance as anode material for lithium ion batteries was firstly investigated. Pure γ-LiFeO2 without nanosizing or carbon coating can deliver the first discharge capacity of 1055.3 mAh/g, and 611.5 mAh/g can be maintained after 50 cycles, around 80 % of the second discharge capacity. γ-LiFeO2 also demonstrates nice rate capabilities. These results indicate that γ-LiFeO2 is a promising anode material candidate for lithium ion batteries. The charge/discharge mechanism was also investigated.









Similar content being viewed by others
Notes
Almost all the literature described the space group of α-LiFeO2 as Fm3m, however, Fm-3m should be the right assignment after careful check of the data from inorganic crystal structure data (ICSD), Pearson’s crystal data (PCD) and related literature
References
Trogadas P, Ramani V, Strasser P, Fuller TF, Coppens MO (2015) Hierarchically structured nanomaterials for electrochemical energy conversion. Angew Chem Int Ed 54:122–148
Vlad A, Singh N, Galande C, Ajayan PM (2015) Design considerations for unconventional electrochemical energy storage architectures. Adv Energy Mater 5:201402115
Hu CG, Song L, Zhang ZP, Chen N, Feng ZH, Qu LT (2015) Tailored graphene systems for unconventional applications in energy conversion and storage devices. Energy Environ Sci 8:31–54
Wu NT, Zhang Y, Guo Y, Liu SJ, Liu H, Wu H (2016) Flakelike LiCoO2 with exposed 010 facets as a stable cathode material for highly reversible lithium storage. ACS Appl Mater Interfaces 8:2723–2731
He P, Yu HJ, Li D, Zhou HS (2012) Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J Mater Chem 22:3680–3695
Kang B, Ceder G (2009) Battery materials for ultrafast charging and discharging. Nature 458:190–193
Barpanda P, Nishimura S, Yamada A (2012) High-voltage pyrophosphate cathodes. Adv Energy Mater 2:201100772
Yan MY, Zhang GB, Mai LQ (2016) In operando observation of temperature dependent phase evolution in lithium-incorporation olivine cathode. Nano Energy 22:406–413
Zhang L, Xiang HF, Wang HH (2012) Synthesis of LiFePO4/C composite as a cathode material for lithium-ion battery by a novel two-step method. J Mater Sci 47:3076–3081
Muruganantham R, Sivakumar M, Subadevi R, Wu NL (2015) A facile synthesis and characterization of LiFePO4/C using simple binary reactants with oxalic acid by polyol technique and other high temperature methods. J Mater Sci Mater Electron 26:2095–2106
Vucinic-Vasic M, Antic B, Blanusa J, Rakic S, Kremenović A, Nikolic AS, Kapor A (2006) Formation of nanosize Li-ferrites from acetylacetonato complexes and their crystal structure, microstructure and order-disorder phase transition. Appl Phys A 82:49–54
Barré M, Catti M (2009) Neutron diffraction study of the β’ and γ Phases of LiFeO2. J Solid State Chem 182:2549–2554
Chappel E, Holzapfel M, Chouteau G, Ott A (2000) Effect of cobalt on the magnetic properties of the LiFe1−xCoxO2 layered system (0 ≤ x ≤ 1). J Solid State Chem 154:451–459
Hirayama M, Tomita H, Kubota K, Kanno R (2011) Structure and electrode reactions of layered rocksalt LiFeO2 nanoparticles for lithium battery cathode. J Power Sources 196:6809–6814
Li JG, Li JJ, Luo J, Wang L, He XM (2011) Recent advances in the LiFeO2-based materials for Li-ion batteries. Int J Electrochem Sci 6:1550–1561
Catti M, Montero-Campillo M (2011) Lithium diffusion pathways and vacancy formation in the Pmmn Li1−xFeO2 electrode material. Phys Chem Chem Phys 13:11156–11164
Armstrong AR, Tee DW, Mantia FL, Novák P, Bruce PG (2008) Synthesis of tetrahedral LiFeO2 and its behavior as a cathode in rechargeable lithium batteries. J Am Chem Soc 130:3554–3559
Jiang J, Li YY, Liu JP, Huang XT, Yuan CZ, Lou XW (2012) Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv Mater 24:5166–5180
Yu XG, Marks TJ, Facchetti A (2016) Metal oxides for optoelectronic applications. Nature Mater 15:383–396
Zhou H, Ruther RE, Adcock J, Zhou W, Dai S, Nanda J (2015) Controlled formation of mixed nanoscale domains of high capacity Fe2O3–FeF3 conversion compounds by direct fluorination. ACS Nano 9:2530–2539
An QY, Lv F, Liu QQ, Han CH, Zhao KN, Sheng JZ, Wei QL, Yan MY, Mai LQ (2014) Amorphous vanadium oxide matrixes supporting hierarchical porous Fe3O4/graphene nanowires as a high-rate lithium storage anode. Nano Lett 14:6250–6256
Du DJ, Yue WB, Ren Y, Yang XJ (2014) Fabrication of graphene-encapsulated CoO/CoFe2O4 composites derived from layered double hydroxides and their application as anode materials for lithium-ion batteries. J Mater Sci 49:8031–8039
Büyükyazi M, Mathur S (2015) 3D nanoarchitectures of α-LiFeO2 and α-LiFeO2/C nanofibers for high power lithium-ion batteries. Nano Energy 13:28–35
Obrovac MN, Dunlap RA, Sanderson RJ, Dahn JR (2001) The electrochemical displacement reaction of lithium with metal oxides. J Electrochem Soc 148:A576–A588
Krummacher J, Passerini S, Balducci A (2015) Ionic liquid assisted solid-state synthesis of lithium iron oxide nanoparticles for rechargeable lithium ion batteries. Solid State Ionics 280:37–43
Rahman MM, Wang JZ, Hassan MF, Chen ZX, Liu HK (2011) Synthesis of carbon coated nano crystalline porous α-LiFeO2 composite and its application as anode for the lithium ion battery. J Alloys Compd 509:5408–5413
Li KY, Chen H, Shua FF, Xue DF, Guo XW (2014) Facile synthesis of iron-based compounds as high performance anode materials for Li-ion batteries. RSC Adv 4:36507–36512
Acknowledgements
We gratefully acknowledge the financial support by National Natural Science Foundation of China (Grant No. 21173183), the Higher Education Science Foundation of Jiangsu Province (No. 15KJB150031), State Key Laboratory of Structural Chemistry Fund (No. 20150009), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Qing Lan project. We would also like to acknowledge the technical support received from the Testing Center of Yangzhou University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, SP., Ma, Z., Li, JC. et al. First investigation of the electrochemical performance of γ-LiFeO2 micro-cubes as promising anode material for lithium-ion batteries. J Mater Sci 52, 1469–1476 (2017). https://doi.org/10.1007/s10853-016-0441-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0441-3