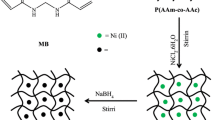
Abstract
Thermosensitive hydrogels displaying a high degree of swelling were successfully fabricated from cross-linked poly(N-isopropylacrylamide-co-2-acrylamido-2-methylpropanesulfonic acid) (P(NIPAM-co-AMPS)). The sulfonic acid groups in the copolymer networks strongly modulated the internal structure and swelling behavior of the P(NIPAM-co-AMPS), thereby lowering the critical solution temperature of the hydrogels. These groups acted as adsorption sites, enabling the in situ synthesis of Ni nanoparticles in P(NIPAM-co-AMPS) hydrogels. Significant changes in the average diameter and number of the Ni nanoparticles were observed when the concentration of AMPS was changed. The catalytic capability of P(NIPAM-co-AMPS)/Ni to act as a switched temperature reactor was demonstrated in a model reduction reaction. Kinetic data revealed that the adsorption of the reactants on the catalyst surface was the key factor in terms of the Langmuir–Hinshelwood model. The reaction rate of the P(NIPAM-co-AMPS)/Ni composite hydrogels did not increase monotonically; instead, the reaction rate decreased to some extent around the low critical solution temperature (LCST), demonstrating a thermally tunable catalytic activity. The P(NIPAM-co-AMPS)/Ni composites were also highly stable, and were usable after five cycles of use, retaining a high level of activity.












Similar content being viewed by others
References
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
Campelo JM, Luna D, Luque R et al (2009) Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2:18–45
Standley EA, Tasker SZ, Jensen KL et al (2015) Nickel catalysis: synergy between method development and total synthesis. Acc Chem Res 48:1503–1514
Dhakshinamoorthy A, Garcia H (2012) Catalysis by metal nanoparticles embedded on metal-organic frameworks. Chem Soc Rev 41:5262–5284
Zhang AQ, Cai LJ, Sui L et al (2013) Reducing properties of polymers in the synthesis of noble metal nanoparticles. Polym Rev 53:240–276
Zhang C, Chao L, Chen Y et al (2014) Synthesis and catalysis of Ag nanoparticles trapped into temperature-sensitive and conductive polymers. J Mater Sci 49:6872–6882. doi:10.1007/s10853-014-8389-7
Cao T, Guo X, Zhou X et al (2011) Synthesis and properties of organic-inorganic hybrid P(NIPAM-co-AM-co-TMSPMA) microgels. Chin J Polym Sci 29:439–449
Song YJ, Jesús YMLD, Fanson PT et al (2014) Kinetic evaluation of direct NO decomposition and NO–CO reaction over dendrimer-derived bimetallic Ir–Au/Al2O3 catalysts. Appl Catal B 154–155:62–72
Narayanan R, El-Sayed MA (2005) Catalysis with transition metal nanoparticles in colloidal solution: nanoparticle shape dependence and stability. J Phys Chem B 109:12663–12676
Sahiner N, Ozay O, Inger E et al (2011) Superabsorbent hydrogels for cobalt nanoparticle synthesis and hydrogen production from hydrolysis of sodium boron hydride. Appl Catal B 102:201–206
Mohan YM, Lee K, Premkumar T et al (2007) Hydrogel networks as nanoreactors: a novel approach to silver nanoparticles for antibacterial applications. Polymer 48:158–164
Yamauchi H, Maeda Y (2007) LCST and UCST behavior of poly(N-isopropylacrylamide) in DMSO/water mixed solvents studied by IR and micro-Raman spectroscopy. J Phys Chem B 111:12964–12968
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49:1993–2007
Billiet T, Vandenhaute M, Schelfhout J et al (2012) A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 33:6020–6041
Sahiner N, Ozay H, Ozay O et al (2010) A soft hydrogel reactor for cobalt nanoparticle preparation and use in the reduction of nitrophenols. Appl Catal B 101:137–143
Liu B, Wang T, Yin C et al (2014) Electrochemical analysis of p-nitrophenol in acidic or alkaline medium using silver nanoparticle decorated multi-walled carbon nanotubes. J Mater Sci 49:5398–5404. doi:10.1007/s10853-014-8251-y
Carregal-Romero S, Buurma NJ, Pérez-Juste J et al (2010) Catalysis by Au@pNIPAM nanocomposites: effect of the cross-linking density. Chem Mater 22:3051–3059
Zhu CH, Hai ZB, Cui CH et al (2012) In situ controlled synthesis of thermosensitive poly(N-isopropylacrylamide)/Au nanocomposite hydrogels by gamma radiation for catalytic application. Small 8:930–936
Shi S, Wang Q, Wang T et al (2014) Thermo-, pH-, and light-responsive poly(N-isopropylacrylamide-co-methacrylic acid)–Au hybrid microgels prepared by the in situ reduction method based on Au-thiol chemistry. J Phys Chem B 118:7177–7186
Schilli CM, Zhang M, Rizzardo E et al (2004) A new double-responsive block copolymer synthesized via RAFT polymerization: poly(N-isopropylacrylamide)-block-poly(acrylic acid). Macromolecules 37:7861–7866
Sahiner N (2006) In situ metal particle preparation in cross-linked poly(2-acrylamido-2-methyl-1-propansulfonic acid) hydrogel networks. Colloid Polym Sci 285:283–292
Chen L, Gong JP, Osada Y (2002) Novel thermosensitive IPN hydrogel having a phase transition without volume change. Macromol Rapid Commun 23:171–174
Kato N, Sakai Y, Shibata S (2003) Wide-range control of deswelling time for thermosensitive poly(N-isopropylacrylamide) gel treated by freeze-drying. Macromolecules 36:961–963
Karlsson LE, Jannasch P, Wesslén B (2002) Preparation and solution properties of amphiphilic sulfonated acrylamide copolymers. Macromol Chem Phys 203:686–694
Sousa RG, Magalhães WF, Freitas RF (1998) Glass transition and thermal stability of poly(N-isopropylacrylamide) gels and some of their copolymers with acrylamide. Polym Degrad Stab 61:275–281
Jana NR, Gearheart L, Murphy CJ (2001) Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 17:6782–6786
Henglein A, Radiolytic (1999) Preparation of ultrafine colloidal gold particles in aqueous solution: optical spectrum, controlled growth, and some chemical reactions. Langmuir 15:6738–6744
Henglein A, Meisel D (1998) Radiolytic control of the size of colloidal gold nanoparticles. Langmuir 14:7392–7396
Cushing BL, Kolesnichenko VL, O’Connor CJ (2004) Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev 104:3893–3946
Rioux R, Song H, Hoefelmeyer J et al (2005) High-surface-area catalyst design: synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J Phys Chem B 109:2192–2202
Liang M, Su R, Qi W et al (2014) Synthesis of well-dispersed Ag nanoparticles on eggshell membrane for catalytic reduction of 4-nitrophenol. J Mater Sci 49:1639–1647. doi:10.1007/s10853-013-7847-y
Esumi K, Isono R, Yoshimura T (2004) Preparation of PAMAM− and PPI− metal (silver, platinum, and palladium) nanocomposites and their catalytic activities for reduction of 4-nitrophenol. Langmuir 20:237–243
Wunder S, Polzer F, Lu Y et al (2010) Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J Phys Chem C 114:8814–8820
Gu S, Wunder S, Lu Y et al (2014) Kinetic analysis of the catalytic reduction of 4-nitrophenol by metallic nanoparticles. J Phys Chem C 118:18618–18625
Sahiner N, Ozay H, Ozay O et al (2010) New catalytic route: hydrogels as templates and reactors for in situ Ni nanoparticle synthesis and usage in the reduction of 2- and 4-nitrophenols. Appl Catal A 385:201–207
Firouzabadi H, Iranpoor N, Gholinejad M et al (2011) Agarose hydrogel as an effective bioorganic ligand and support for the stabilization of palladium nanoparticles. Application as a recyclable catalyst for Suzuki-Miyaura reaction in aqueous media. RSC Adv 1:1013–1019
Kuroda K, Ishida T, Haruta M (2009) Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J Mol Catal A 298:7–11
Wang A, Yin H, Lu H (2009) Catalytic activity of nickel nanoparticles in hydrogenation of p-nitrophenol to p-aminophenol. Catal Commun 10:2060–2064
Blosi M, Albonetti S, Costa A et al (2013) Easily scalable synthesis of Ni nanosols suitable for the hydrogenation of 4-nitrophenol to p-aminophenol under mild condition. Chem Eng J 215–216:616–625
Lu H, Yin H, Liu Y et al (2008) Influence of support on catalytic activity of Ni catalysts in p-nitrophenol hydrogenation to p-aminophenol. Catal Commun 10:313–316
Saha S, Pal A, Kundu S (2009) Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 26:2885–2893
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grants Nos. 21374078 and 21174103).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Youzhi, G., Yang, H., Yiping, Z. et al. Fabrication of thermosensitive hydrogel-supported Ni nanoparticles with tunable catalytic activity for 4-nitrophenol. J Mater Sci 51, 3200–3210 (2016). https://doi.org/10.1007/s10853-015-9631-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9631-7