Abstract
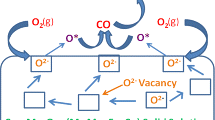
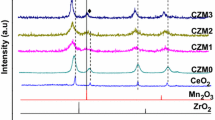
The effect of composition on the thermal stability and oxygen storage capacity (OSC) of a series of CeO2–MOx (M: Zr, Ti, Cu) mixed oxides was investigated. X-ray diffraction, N2 adsorption–desorption analysis (BET), transmission electron microscopy, Raman spectroscopy, and X-ray photoelectron spectroscopy were used to examine structural and microstructural properties. The total OSC of the fresh and aged mixed oxides were measured using thermogravimetric–differential thermal analysis at 600 °C, under alternating reductive and oxidative environment. Ceria doping was found to be effective toward an enhancement of its redox properties. The addition of Zr4+ into the CeO2 lattice was found to have the least effect in enhancing the catalytic properties. In contrast with other studies in the literature [1, 2], increasing ZrO2 content led to an increase of the OSC of the samples of up to 522 μmol O2 g−1 for 75 % Zr4+ content. On the other hand, the CeO2–CuO with (1:3) molar ratio showed an almost three times higher OSC value than CeO2–ZrO2, which is deemed in the literature as one of the most promising OSC materials. In this work, the highest OSC values (1565 μmol-O2 g−1) were obtained for the fresh sample with CeO2–CuO (25:75) composition. The CeO2–CuO (25:75) mixed oxide still showed the highest OSC values with an only 6 % drop of OSC after aging at 900 °C for 6 h among all aged catalysts. On the other hand, ZrO2 containing samples showed the best thermal resistance against aging.












Similar content being viewed by others
References
Trovarelli A, Boaro M, Rocchini E, de Leitenburg C, Dolcetti G (2001) Some recent developments in the characterization of ceria-based catalysts. J Alloys Compd 323:584–591
Sugiura M, Ozawa M, Suda A, Suzuki T, Kanazawa T (2005) Development of innovative three-way catalysts containing ceria-zirconia solid solutions with high oxygen storage/release capacity. Bull Chem Soc Jpn 78(5):752–767
Dong Q, Yin S, Guo CS, Sato T (2012) Ce0.5Zr0.4Sn0.1O2/Al2O3 catalysts with enhanced oxygen storage capacity and high CO oxidation activity. Catal Sci Technol 2(12):2521–2524
Fally F, Perrichon V, Vidal H, Kaspar J, Blanco G, Pintado JM, Bernal S, Colon G, Daturi M, Lavalley JC (2000) Modification of the oxygen storage capacity of CeO2-ZrO2 mixed oxides after redox cycling aging. Catal Today 59(3–4):373–386
Benjaram MR, Gode T, Katta L (2011) Nanosized unsupported and alumina-supported ceria-zirconia and ceria-terbia solid solutions for co oxidation. Chin J Catal 32(5):800–806
Jiang L, Zhu HW, Razzaq R, Zhu ML, Li CS, Li ZX (2012) Effect of zirconium addition on the structure and properties of CuO/CeO2 catalysts for high-temperature water-gas shift in an IGCC system. Int J Hydrog Eng 37(21):15914–15924
Dong Q, Yin S, Guo CS, Sato T (2012) A new oxygen storage capacity material of a tin-doped ceria-zirconia-supported palladium-alumina catalyst with high CO oxidation activity. Chem Lett 41(10):1250–1252
Huang P, Jiang HX, Zhang MH (2012) Structures and oxygen storage capacities of CeO2-ZrO2-Al2O3 ternary oxides prepared by a green route: supercritical anti-solvent precipitation. J Rare Earths 30(6):524–528
Zhu LY, Wang XQ, Ren Q, Zhang GH, Xu D (2012) Morphology and crystal structure of CeO2-modified mesoporous ZrO2 powders prepared by sol-gel method. Mater Chem Phys 133(1):445–451
Wang H-F, Li H-Y, Gong X-Q, Guo Y-L, Lu G-Z, Hu P (2012) Oxygen vacancy formation in CeO2 and Ce1-xZrxO2 solid solutions: electron localization, electrostatic potential and structural relaxation. Phys Chem Chem Phys 14(48):16521–16535
Ozawa M, Yuzuriha H, Haneda M (2013) Total oxidation of toluene and oxygen storage capacity of zirconia-sol modified ceria zirconia. Catal Commun 30:32–35
Tschope A, Ying JY (1994) Nanocrystalline cerium oxide catalytic materials. Nanophase Mat 260:781–784
Zhang Y, Andersson S, Muhammed M (1995) Nanophase catalytic oxides. 1. Synthesis of doped cerium oxides as oxygen storage promoters. Appl Catal B 6(4):325–337
Wu XD, Qing L, Duan W (2007) Role of surface adsoption in fast oxygen storage/release of CeO2–ZrO2 mixed oxides. J Rare Earths 25(4):416–421
Abdollahzadeh-Ghom S, Zamani C, Andreu T, Epifani M, Morante JR (2011) Improvement of oxygen storage capacity using mesoporous ceria-zirconia solid solutions. Appl Catal B 108(1–2):32–38
Schmieg SJ, Belton DN (1995) Effect of hydrothermal aging on oxygen storage release and activity in a commercial automotive catalyst. Appl Catal B 6(2):127–144
Epifani M, Andreu T, Abdollahzadeh-Ghom S, Arbiol J, Morante JR (2012) Synthesis of ceria-zirconia nanocrystals with improved microstructural homogeneity and oxygen storage capacity by hydrolytic sol-gel process in coordinating environment. Adv Funct Mater 22(13):2867–2875
Li GF, Wang QY, Zhao B, Zhou RX (2012) A new insight into the role of transition metals doping with CeO2–ZrO2 and its application in Pd-only three-way catalysts for automotive emission control. Fuel 92(1):360–368
Bunluesin T, Gorte RJ, Graham GW (1997) CO oxidation for the characterization of reducibility in oxygen storage components of three-way automotive catalysts. Appl Catal B 14(1–2):105–115
Gorte RJ (2010) Ceria in catalysis: from automotive applications to the water gas shift reaction. AIChE J 56(5):1126–1135
Cordatos H, Bunluesin T, Stubenrauch J, Vohs JM, Gorte RJ (1996) Effect of ceria structure on oxygen migration for Rh/ceria catalysts. J Phys Chem 100(2):785–789
Ouyang J, Yang HM (2009) Investigation of the oxygen exchange property and oxygen storage capacity of CexZr1-xO2 nanocrystals. J Phys Chem C 113(17):6921–6928
Chuang CC, Hsiang HI, Hwang JS, Wang TS (2009) Synthesis and characterization of Al2O3-Ce0.5Zr0.5O2 powders prepared by chemical coprecipitation method. J Alloy Compd 470(1–2):387–392
Rabelo NRC, Martin S (2012) Synthesis of CeO2 and CeZrO2 mixed oxide nanostructured catalysts for the iso-syntheses reaction. Appl Catal A 450:131–142
Zhou GL, Lan H, Yang XQ, Du QX, Xie HM, Fu M (2013) Effects of the structure of Ce-Cu catalysts on the catalytic combustion of toluene in air. Ceram Int 39(4):3677–3683
Zhou G, Shah PR, Montini T, Fornasiero P, Gorte RJ (2007) Oxidation enthalpies for reduction of ceria surfaces. Surf Sci 601(12):2512–2519
Choudhury B, Choudhury A (2012) Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater Chem Phys 131(3):666–671
Choudhury B, Choudhury A (2013) Lattice distortion and corresponding changes in optical properties of CeO2 nanoparticles on Nd doping. Curr Appl Phys 13(1):217–223
Reddy MB, Khan A (2005) Nanosized CeO2-SiO2, CeO2–TiO2, and CeeO2-ZrO2 mixed oxides: influence of supporting oxide on thermal stability and oxygen storage properties on ceria. Catal Surv Asia 9:151–171
Kim J-R, Myeong W-J, Ihm S-K (2009) Characteristics of CeO2–ZrO2 mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as support of Rh catalyst for catalytic reduction of NO by CO. J Catal 263(1):123–133
Graham GW, Weber WH, Peters CR, Usmen R (1991) Empirical-method for determining CeO2-particle size in catalysts by Raman-spectroscopy. J Catal 130(1):310–313
Yue L, Zhang X (2008) Preparation of highly dispersed CeO2/TiO2 core-shell nanoparticles. Mater Lett 62(21–22):3764–3766
Shan WP, Liu FD, He H, Shi XY, Zhang CB (2012) An environmentally-benign CeO2–TiO2 catalyst for the selective catalytic reduction of NOx with NH3 in simulated diesel exhaust. Catal Today 184(1):160–165
Bueno-Ferrer C, Parres-Esclapez S, Lozano-Castello D, Bueno-Lopez A (2010) Relationship between surface area and crystal size of pure and doped cerium oxides. J Rare Earths 28(5):647–653
Weng XL, Perston B, Wang XZ, Abrahams I, Lin T, Yang SF, Evans JRG, Morgan DJ, Carley AF, Bowker M et al (2009) Synthesis and characterization of doped nano-sized ceria-zirconia solid solutions. Appl Catal B Environ 90(3–4):405–415
Liu W, Flytzani-Stephanopoulos M (1995) Total oxidation of carbon-monoxide and methane over transition metal-fluorite oxide composite catalysts. 2. Catalyst characterization and reaction-kinetics. J Catal 153(2):317–332
Liu ZG, Xie YL, Li WS, Zhou RX, Zheng XM (2011) Influence of reduction energy match among CuO species in CuO-CeO2 catalysts on the catalytic performance for CO preferential oxidation in excess hydrogen. J Nat Gas Chem 20(2):111–116
Wang Z, Qu ZP, Quan X, Li Z, Wang H, Fan R (2013) Selective catalytic oxidation of ammonia to nitrogen over CuO-CeO2 mixed oxides prepared by surfactant-templated method. Appl Catal B 134:153–166
Maupin I, Mijoin J, Barbier J, Bion N, Belin T, Magnoux P (2011) Improved oxygen storage capacity on CeO2/zeolite hybrid catalysts. Application to VOCs catalytic combustion. Catal Today 176(1):103–109
Huang LH, Chen SH, Zhang QL, Gong MC, Chen YQ (2013) Influence of composition of ageing system on performance of Ce0.65Zr0.35O2 oxygen storage materials. Acta Phys Chim Sin 29(5):1097–1106
Zhang M, Jiang D, Jiang H (2012) Enhanced oxygen storage capacity of Ce0.88Mn0.12Oy compared to CeO2: an experimental and theoretical investigation. Mater Res Bull 47(12):4006–4012
Wang JQ, Shen MQ, Wang J, Gao JD, Ma J, Liu SX (2011) CeO2-CoOx mixed oxides: structural characteristics and dynamic storage/release capacity. Catal Today 175(1):65–71
Wang JQ, Zhang BY, Shen MQ, Wang J, Wang WL, Ma J, Liu SX, Jia LW (2011) Effects of Fe-doping of ceria-based materials on their microstructural and dynamic oxygen storage and release properties. J Sol Gel Sci Technol 58(1):259–268
Wang DY, Kang YJ, Doan-Nguyen V, Chen J, Kungas R, Wieder NL, Bakhmutsky K, Gorte RJ, Murray CB (2011) Synthesis and oxygen storage capacity of two-dimensional ceria nanocrystals. Angew Chem Int Ed 50(19):4378–4381
Wang JQ, Shen MQ, Wang J, Yang M, Wang WL, Ma J, Jia LW (2010) Effects of Ni-doping of ceria-based materials on their micro-structures and dynamic oxygen storage and release behaviors. Catal Lett 140(1–2):38–48
Sugiura M (2003) Oxygen storage materials for automotive catalysts: ceria-zirconia solid solutions. Catal Surv Asia 7(1):77–87
Acknowledgements
The authors would like acknowledge financial support by NSF under Award No. CMMI 1200066. The XPS data were obtained by the Surface Analysis Laboratory of the Birck Nanotechnology Center, Purdue University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uzunoglu, A., Zhang, H., Andreescu, S. et al. CeO2–MO x (M: Zr, Ti, Cu) mixed metal oxides with enhanced oxygen storage capacity. J Mater Sci 50, 3750–3762 (2015). https://doi.org/10.1007/s10853-015-8939-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8939-7