Abstract
A glass-forming domain is found and studied within Bi2O3–Sb2O3–TeO2 system. The glasses composition were obtained in pseudo-binary xSbO1.5, (1−x)TeO2 for 0.05 ≤ x ≤ 0.20. The constitution of glasses in the system Sb2O3–TeO2 was investigated by DSC, Raman, and Infrared spectroscopy. The influence of a gradual addition of the modifier oxides on the coordination geometry of tellurium atoms has been elucidated based Infrared and Raman studies and showed the transition of TeO4, TeO3+1, and TeO3 units with increasing Sb2O3 content. XRD results reveal the presence of three crystalline: γ-TeO2, α-TeO2, and SbTe3O8 phases during the crystallization process. The density of glasses has been measured. The investigation in the ternary system by the solid state reaction using XRD reveals the existence of a solid solution Bi1−xSb1−xTe2xO4 isotopic to BiSbO4 with 0 ≤ x ≤ 0.1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tellurium dioxide (TeO2) is an important material in both amorphous as well as crystalline form and finds application in active optical devices in particular, a huge hyper-susceptibility, deflectors, modulators, γ-ray detectors, and gas sensors because of its high acousto-optic figure of merit, chemical stability, and mechanical durability [1–6]. It is also not hygroscopic and has superior physical properties such as high dielectric constant and low melting point (800 °C) [7–10]. The origin of the extraordinary non-linear optical properties of TeO2-based glasses is attributed to high hyperpolarizability of a lone electron pair related to the 5 s orbital of tellurium atom. Presently, the well-recognized three modifications of crystalline TeO2 are α-TeO2, β-TeO2, and γ-TeO2 [11–16]. Of these, recently documented γ-TeO2 phase has gained a lot of attention for nonlinear optical designs and efforts are made to understand its properties in bulk crystal and glass using as Fourier transform infra-red spectroscopy (FTIR) and Raman spectroscopy. It has been reported that γ-TeO2 phase appears as the first crystalline structure during the temperature-induced crystallization of TeO2 glass [17–20]. TeO2 glass is not stable. An addition of second oxide component MnOm makes glasses structures more stable [21–28].
In the present study, we have revisited this system in one hand to obtain more information on the structure of these glasses reported by Charton et al. in Sb2O4–TeO4 system [29, 30]. On the other hand we report the formation, the thermal properties and the local structure using Infrared and Raman studies of glasses prepared in the TeO2–Sb2O3 pseudo-binary and crystalline phases in ternary system. A detailed analysis of the crystalline phase formation in this system synthesized in an oxygen flow will be described successively also.
Experimental procedure
The amorphous and crystalline samples were prepared using high purity commercial materials Bi2O3, TeO2 Sb2O3 of analytical grade (Aldrich 99.9%). The batches of suitable proportions of starting products were mixed in an agate mortar and then heated in air at 800 °C (20 min) for vitreous phases and at 600–800 °C (48 h) for crystalline phases. All of them are quenched to room temperature and identified by X-ray diffraction (XRD) using a Bruker D8 Advance diffractometer (Cu-K-alpha radiation). Tg (glass temperature) and Tc (crystallization temperature) were determined using Differential Scanning Calorimetry (DSC) Netsch 2000 PC type from powder samples glasses for about 8 mg in aluminum pans. A heating rate of 10 °C/min was used in the 30–600 °C range. Infrared absorption measurements between 2,000 and 400 cm−1 were made for powder specimens dispersed in a pressed KBr disk. The Raman spectra were recorded in the 80–1,000 cm−1 range using a Jobin–Yvon spectrometer (64000 model) equipped with an Ar + laser (514.5 nm exciting line) and a CCD detector in a backscattering geometry. The sample focalization was controlled through a microscope (×100). The diameter of the laser spot focused on the sample was about 1 mm. The spectra were recorded in two scans (during 100 s) at low power (<100 mW) of the excitation line, in order to avoid damage of the glasses. The spectral resolution was about 2.5 cm−1 at the exciting line. The densities of samples were measured according to the Archimedes principle using diethyl orthophthalate as liquid.
Results and discussion
A wide range glass system based on the Bi2O3–Sb2O3–TeO2 system was prepared at 800 °C. This temperature has been chosen to have a homogenous reagent in one hand and to avoid volatilization of TeO2 at high temperature (\( T_{{{\text{TeO}}_{ 2} }} \)Melting = 732 °C) on the other hand (Fig. 1). The color of the glass changes slightly from dark yellow to yellow with increasing Sb2O3 and Bi2O3 concentration. The glassy domain obtained interval in the pseudo-binary xSbO1.5, (1−x)TeO2 0.05 ≤ x ≤ 0.20 is slightly different to the one proposed by Charton et al. in the xSb2O4, (1−x)TeO2 system study where 0.02 ≤ x ≤ 0.175 [29, 30].
Differential scanning calorimetry
Series of glasses composition are listed in Table 1. An addition of SbO1.5 (up to 5 mol%) would result in the increase of glass stability (as indicated by Tc − Tg). This is presumably due to the participation of Sb3+/Sb5+ in the glass network. The values of Tg, \( T_{{{\text{c}}_{1} }} \), \( T_{{{\text{c}}_{2} }} \), and \( T_{{{\text{c}}_{3} }} \) are presented (Fig. 2 and Table 1).
The DSC curves exhibit an endothermic effect due to glass transition (Tg). At higher temperatures three exothermic peaks are observed and related to temperature crystalline phases. Figure 2 shows the dependence of characteristic temperature, glass transition, the first crystallization (\( T_{{{\text{c}}_{1} }} \)), the second (\( T_{{{\text{c}}_{2} }} \)), and the third crystallization (\( T_{{{\text{c}}_{3} }} \)) on Sb2O3 content. The appearance of single peak (all glasses) due the glass transition temperature indicates the homogeneity of the glasses prepared. With increasing in the concentration of Sb2O3 in the glass matrix, the Tg increases and the difference (Tc − Tg) (about 77–98 °C) implies a thermal stability of glasses. In a study of alkali tellurite glasses, Pye et al [31] showed that the temperature of the glass transition decreases with increasing amount of Li, Na, or K compound. The dependence of Sb2O3 content shows a different tendency especially of glass transition compared with the alkali tellurite glasses. The alkali atoms easily move in the glass structure. However, antimony atoms move with greater difficulty in the glass, because the Sb atom is restrained by relatively strong bonds to every coordinate oxygen. The slight change of the temperature of crystallization of a vitreous composition to another is due to the kinetic phenomenon. Based on XRD and DSC analysis for glassy samples 5–20 mol% SbO1.5 (see Fig. 3) a first peak of crystallization corresponds to the γTeO2, αTeO2, and SbTe3O8 at 405–437 °C range. This phenomenon which we observed the crystallization γTeO2 variety could be expected: similar behavior has been observed in the many others systems as TeO2–WO3 [15], Nb2O5–TeO2 [13, 16], TeO2–ZnO [18] and TeO2–SrO [19]. In second crystallization ranging 497–519 °C belongs to reinforcing SbTe3O8 and TeO2α phases. The last peak (563–573 °C) with weak intensity is attributed to totally transformation γTeO2 metastable polymorph into the stable αTeO2 and SbTe3O8. It can be observed that there is a linear relationship between Tc − Tg and Tc against the Sb2O3 content. This indicates that the glass is easily fabricated. The increase in glass stability is also reported to be due to the structural formation of SbTe3O8 units.
Density
The density of the specimens was measured using Archimeds principle using orthophtalate as the immersion liquid (dorthophtalate = 1.11712 at 22 °C). A glass disc was weighted in air (Wair) and immersed in orthophtalate and reweighted (Worthophtalate). The relative density is given by the following relation [22]:
In pseudo-binary, the glass density increases with the augmentation of SbO1.5/TeO2.
From the result (see Table 1), it can be seen that values of density increase with the addition of Sb2O3 is obviously due the difference of Sb and Te atoms weights.
Infrared spectroscopy
The infrared spectra transmission of glasses compositions are given in Fig. 4. A tellurite network basically consists of TeO4 trigonal bipyramids (TBP) units and TeO3 trigonal pyramid (TP) units, each of which has a lone pair of electrons, while the constitution of binary glasses depends on the second metal oxides. Suzuki [33] reported that TBP units were converted to TP ones on barium and sodium tellurite glasses. He proposed a mechanism (Eqs. 1 and 2) of the formation of the TP units:
where O1/2 represents bridging oxygen. These two reactions proceed as the content of a network modifying oxide increases until all the oxygen atoms of the (TBP) units become non-bridging (Eq. 3).
The three oxygens in the TeO3 2− units are equivalent.
The TeO2 glass (x = 0) infrared spectrum is rather similar to αTeO2 data including the typical broadening observed in glasses. TeO2 vitrification is thus characterized by a redistribution of infrared intensities due to spatial rearrangement of TeO4E units involving a decrease of TeO4E units symmetry which explains that the band at 625 cm−1 becomes predominant [33]. As the Sb2O3 proportion increases (Fig. 4), the major band shifts from 625 cm−1(x = 0) to 667 cm−1 (x > 0.05) which is attributed to TeO3E trigonal pyramid. The presence of shoulder around 780 cm−1 for all studied glass compositions is the signature of TeO4E trigonal pyramid. For x = 0.20 we observed a band at 750 cm−1 nearly which attributed to TeO3+1 group. The absorption band nearly 500 cm−1 which slightly increases in intensity with Sb2O3 content can be assigned to Te–O–Te and Te–O–Sb bridging bonds which would increase the network connectivity in agreement with th Tg increase. On the other hand, from the reference spectra lithium tellurite glasses the infrared broad absorption sharp bands at around 610 cm−1 are attributed to group vibration of TeO6 [21, 34]; Therefore in our preparation glasses, we do not observed this band so there is no oxidation of Te+4 to Te+6.
Raman spectra
In tellurite glasses, XANES, X-ray absorption and Raman spectroscopy previously showed that tellurium is surrounded by oxygen atoms and generally localized in three types of sites with different geometries. For the lowest amounts of additional oxides the dominant tellurium site are [TeO4] trigonal bipyramids which are axially elongated and partly connected to each other sharing one oxygen atom. When increasing the amount of additional oxides they progressively convert into [TeO3] regular trigonal pyramids via [TeO3+1] entities where one axial Te–Oax distance is elongating while the others shortens getting closer to the shortest equatorial Te–Oeq distances [25, 28–30, 35–41].
The Raman spectra of xSbO1.5, (1−x) TeO2 (5 ≤ x ≤ 20% mol) glasses are shown in Fig. 5a. For all samples, spectra obtained from different spots are identical showing high homogeneity of glasses. They are two pronounced peaks occur around 640–670 and 760–770 cm−1. The most prominent band at 659 cm−1 in the spectrum of pure glass is related to the combined vibrations of asymmetric stretching of Teeq–Oax–Te bonds and symmetric stretching of TeO4 (TBP). With addition of SbO1.5 up 20% mol fraction, intensity of this band decreases (G1), while bands at 760–768 cm−1 (G2) attributed to stretching vibrations of non-bridging Te–O– bands in TeO3 (TP). The peak (G2), which is assigned to a stretching vibration of TeO4 units, was observed to decrease as the Sb2O3 contents increases. The decrease in intensity would suggest the possibility of conversion from TeO4 TBP units to the other basic structural unit [37]. The peak (G1) is reported to be due to the perturbation of TeO4 (TBP) units into TeO3 (trigonal pyramids) units via the intermediate coordination of TeO3+1 [34, 35, 37]. Both features would clearly indicate that the network of the TeO3 structural unit increases with the increasing of Sb2O3 contents. Other peaks around (P) 452–456 cm−1 are observed to be less sensitive to the Sb2O3 contents. Antimony atoms are incorporation in the glass implied the formation of Te–O–Sb bridging bonds which stabilizes the glass formation in accordance with Tc − Tg increase. A decrease in the peak intensity would suggest the occurrence of the destruction of Te–O–Te (or O–Te–O) in the linkages, thus resulted in the decreasing of the Te–O–Te linkages in a continue network of TeOn (n = 4, 3 + 1, or 3) entities, which is consistent with the observation reported elsewhere [35], the intensity of this band decreases, while bands at 760 and 769 cm−1 were attributed to stretching vibrations of non-bridging Te–O– bands in TeO3 (TP) grow in intensity.
The spectral deconvolution of binary glasses indicates that five strong bands are present at about 450–767 cm−1. The bands are mainly attributed to the vibrations of coordination polyhedra of tellurium atoms. Figure 5b and Table 2 show results of band deconvolution of the spectra of xSbO1.5, (1−x) TeO2 (5 ≤ x ≤ 20% mol). A good agreement was obtained between the observed and simulated spectra. The fitting of the spectra was made with focus, a curve fitting soft wave especially adapted for analysis of optical spectra [42].
The orthotellurate ion, TeO6 6−, will have octahedral symmetry but may be strongly distorted. Vibrational modes for the tellurate anion should occur in the 620–650 cm−1 and in the 290–360 cm−1 regions [43]. In our spectrum, these intense bands do not appear, therefore there is no Te6 + in our vitreous composition.
Crystalline phases
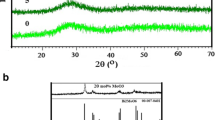
A solid state investigation of the Bi2O3–Sb2O3–TeO2 system allowed synthesis of crystalline phases SbTe3O8 and Sb2Te2O9 which have been obtained at 600–750 °C in air and characterized by XRD.
SbTe3O8
This phase, is characterized by powder diffraction X and indexed in the cubic system with parameter a = 11.025(2) Å. No significant change in weight was observed, result implies no oxidation of TeIV. This phase derived from fluorine phase is seems isotopic to TiTe3O8 [44] and its structure determination well be published.
Sb2Te2O9
This phase is obtained from 2 mol TeO2 and 1 mol Sb2O3. The mass gain during the synthesis of this compound was observed, which is probably related to an increase in oxygen content due to the oxidation of Te4+ to Te6+ and/or Sb3+ to Sb5+. Using the 11 most intense reflections of X-ray diffraction powder pattern, the indexing program dicvol [45] yielded monoclinic symmetry. All observed reflections were indexed and the figures merit were M20 = 38. After a least-squares refinement, the cell parameters were: a = 21.466(1) Å, b = 4.903(1) Å, c = 14.469(1) Å; β = 110.89(1)°. These parameters were good agreement as reported in the ICDD files n°79-2317.
Analysis by diffraction X of Bi2O3–TeO2–Sb2O3(Sb2O5) system
A series of compositions in the system of carefully chosen and were treated at different temperatures between 600 and 800 °C. Their analysis by X-ray diffraction revealed the existence of a stable phase BiSbO4 [46] and other phases localized in Bi2O3–TeO2 or Bi2O3–Sb2O3(Sb2O5) pseudo- binary. Typical X-ray diffraction patterns of Bi1−xSb1−xTe2xO4 0 ≤ x ≤ 0.5 are shown in (Fig. 6). Solid solution of Bi1−xSb1−xTe2xO4 exist for the range (0 ≤ x ≤ 0.1) (compositions B and C) and the lattice parameters from XRD pattern are listed in Table 3. They are compared to BiSbO4 phase. We find that the coupled substitution of antimony and bismuth atoms in size by the average of tellurium in the network and has no significant influence on the evolution of lattice parameters. Phase up to x = 0.5 (BiSbTeO4) (A) adopt the Bi2Te2O7 and the limit solid solution.
Remarks
Owing to the oxidation of Sb+3 and Te+4, the investigated Bi2O3–Sb2O3–TeO2 system cannot be considered as a ternary, but rather as a pseudo-quaternary Bi3+/Te+4/Te+6/Sb+3/Sb+5 system. Our investigation of the Bi2O3–Sb2O3–TeO2 system revealed the important influence of the oxygen atmosphere on the chemistry and the crystallography of the phases found.
Conclusions
In the Bi2O3–Sb2O3–TeO2 pseudo-ternary system, stable and transparent glasses were been synthesized at 800 °C. The vitreous domain in pseudo-binary (1−x)TeO2–xSbO1.5 system is: 0.05 ≤ x ≤ 0.2. Its characteristic temperatures (glass transition and crystallization temperatures) have been determined. The crystallization of the samples rich of TeO2 occurs for the γTeO2, Te3SbO8 and αTeO2. The γTeO2 variety transforms complete to αTeO2 up 500 °C. The IR and Raman spectra of glasses were interpreted in terms of structural transformations produced by modifiers; from TeO4 trigonal bipyramid to TeO3 trigonal pyramid via [TeO3+1] entities with increasing of the Sb2O3 content in glass. The formation Sb–O–Te linkages and 3-fold coordinated oxygen atoms which increase the polymerization of the glass network in accordance with an increase of the glass transition temperature which proportion depends on composition. A solid state investigation by X-ray of the system allowed Te3SbO8, where no oxidation of Te4+ to Te6+ is allowed by synthesizing in oxygen atmosphere and the Sb atoms present is a mixed oxidation state, Sb3+ and Sb5+. In the Sb2Te2O9 phase, the antimony and tellurium atoms are in Sb5+ and Te6+ state, respectively. The densities of the glasses increase in Sb2O3 content. The investigation by X-ray diffraction in the ternary system allowed the existence a solid solution with the formulation Bi1−xSb1−xTe2xO4 isotopic to BiSbO4:0 ≤ x ≤ 0.1.
References
Abdulhalim I, Pannel CN, Wang J, Wylangowski G, Payne DN (1994) J Appl Phys 75:519
Yano T, Fukumoto A, Watanabe A (1971) J Appl Phys 42:3674
Kotov VM, Shkerdin GN, Shkerdin DG, Kotov EV (2005) J Opt Technol 72:511
Takenaga M, Yamada N, Nishiuchi K, Akahira N, Ohta T, Nakamura S, Yamashita T (1983) J Appl Phys 54:5376
Arshak K, Korostynska O (2002) Sensors 2:347
Sen S, Muthe KP, Joshi N, Gadkari SC, Gupta SK, Jagannath M, Roy M, Deshpande SK, Yakhmi JV (2004) Sensors Actuators B 98:154
El-Mallawany R (1992) J Appl Phys 72:1774
Kim SH, Yoko T, Sakka S (1993) J Am Ceram Soc 76:2486
El-Mallawany RAH (2001) Tellurite glasses handbook. CRC Press, Boca Raton, p 113
Nasu H, Matsushita O, Kamiya K, Kobayashi H, Kubodera K (1990) J Non-Cryst Solids 124:275
Thomas PA (1988) J Phys C 21:4611
Beyer VH (1967) Zeitschrift für Kristallographie 124:228
Blanchandin S, Marchet P, Thomas P, Champarnaud-Mesjard J-C, Frit B (1999) J Mater Chem 9:1785
Champarnaud-Mesjard J-C, Blanchandin S, Thomas P, Mirgodsky AP, Merle-Mejean T, Frit B (2000) J Phys Chem Solids 61:1499
Blanchandin S, Thomas P, Marchet P, Frit B, Chagraoui A, Mater J (1999) Sciences 34:4285
Blanchandin S, Thomas P, Marchet P, Champarnaud-Mesjard JC, Frit B (2002) J Alloys Compd 34:206
Dewan N, Sreenivas K, Gupta V (2007) J Cryst Growth 305:237
Chagraoui A, Chakib A, Mandil A, Tairi A, Ramzi Z, Benmokhtar S (2007) Scripta Materialia 56:93
Chagraoui A, Ramzi Z, Tairi A, Mandil A, Talibouridah M, Ajebli K, Abboud Y (2009) J Mater Process Technol 209:3111
Chagraoui A, Bensaid H, Tairi A, Ajebli K, Moussaoui A (2010) J Alloys Compd 495:67
Idalgo E, Ara′ujo EB, Yukimitu K, Moraes JCS, Reynoso VCS, Carvalho CL (2006) Mater Sci Eng A 434:13
Sidek HAA, Hamezan M, Zaidan AW, Talib ZA, Kaida K (2005) Am J Appl Sci 2(8):1266
Sekiya T, Mochida N, Soejima A (1995) J Non-Cryst Solids 19i:115
Rosmawati S, Sidek HAA, Zainal AT, Mohd Zobir H (2008) J Appl Sci 8(10):1956
Rong QJ, Osaka A, Nanba T, Takada J, Miura Y (1992) J Mater Sci 27:3793. doi:https://doi.org/10.1007/BF00545458
Udovic M, Thomas P, Mirgorodsky A, Masson O, Merle-Mejean T, Lasbrugnas C, Champarnaud-Mesjard JC, Hayakawa T (2009) Mater Res Bull 44:248
Chen Y, Nie Q, Xu T, Dai S, Xang X, Shen X (2008) J Non-Cryst Solids 354:3468
Soulis M, Mirgorodsky AP, Merle-Mejean T, Masson O, Thomas P, Udovic M (2008) J Non-Cryst Solids 354:143
Charton P, Armand P (2003) J Non-Cryst Solids 316:189
Charton P, Thomas P, Armand P (2003) J Non-Cryst Solids 321:81
Pye LD, Stevens HJ, Lacourse WC (1992) The physics of non-crystalline solids. Taylor and Francis, London, p 281
Blanchandin S, Thomas P, Marchet P, Champarnaud-Mesjard JC, Frit B (1999) J Mater Chem 9:1785
Suzuki K (1987) J Non-Cryst Solids 95/96:15
Sekiya T, Mochida N, Ohtsuka J, Tonokawa M (1989) Nippon Seramikkusu, Kyokai Gakujutsu Ronbunshi 97:1435
Nazabal V, Todoroki S, Nukui A, Matsumoto T, Suehara S, Hondo T, Araki T, Inoue S, Rivero C, Cardinal T (2003) J Non-Cryst Solids 325:85
Kawasaki S, Honma T, Benino Y, Pujiwara T, Sato R, Komatsu T (2003) J Non-Cryst Solids 325:61
Li H, Su Y, Sundaram SK (2001) J Non-Cryst Solids 293–295:402
Sabadel Armand JC, Cachau-Herreillat D, Baldeck P, Doclot O, Ibanez A, Philippot E (1997) J Solid State Chem 132:411
Komatsu T, Tawarayama H, Mohri H, Matusita K (1991) J Non-Cryst Solids 135:105
Hu L, Jiang Z (1996) Phys Chem Glasses 37(1):19
Dimitrova-Pankova M, Dimitriev Y, Arnaudov M, Dimitrov V (1989) Phys Chem Glasses 30(6):260
Focus web site.<https://doi.org/crmht.cnrs-orleans.fr/pot/software/focus.html>
Frost RL, Keeffe EC (2009) J Raman Spectrosc 40:249
Bindi L, Cipriani C (2003) Can Mineral 41:1469
Boultif A, Louer D (1991) J Appl Crystallogr 24:987
Kennedy, Brendy J (1994) Powder Diffr 9:164
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://doi.org/creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Chagraoui, A., Yakine, I., Tairi, A. et al. Glasses formation, characterization, and crystal-structure determination in the Bi2O3–Sb2O3–TeO2 system prepared in an air. J Mater Sci 46, 5439–5446 (2011). https://doi.org/10.1007/s10853-011-5485-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5485-9