Abstract
The results of X-ray diffraction, scanning electron microscopy, and dielectric measurements performed for polycrystalline Ba(Ti1−x Zr x )O3 are presented. Data from these measurements show a change of the type of paraelectric–ferroelectric (PE–FE) phase transition (PT) from sharp PT (x < 0.10), through diffuse phase transition (0.10 ≤ x ≤ 0.20), to relaxor type one (x = 0.30). The lack of structural PT at the temperature T m (the temperature of the maximum of real part of the electric permittivity ε′) for 0.10 ≤ x ≤ 0.30, suggest that this PE–FE transition is not connected with the change of crystal structure. A phase angle of about Φ ≈ −90°, between electric current and applied voltage, suggests the occurrence of polar regions (clusters) below 400 K. The change of these clusters polarizability and their lability at the T m is postulated as possible origin of the observed maximum of ε′.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The solid solution Ba(Ti1−x Zr x )O3 (BTZx) is the ferroelectric material of perovskite type; A(B′B″)O3. The pure barium titanate (BT) forms four structural phases [1]. At high temperatures (in the paraelectric phase) it has a cubic structure. During decrease of the temperature in BT sample three phase transitions (PT) take place: first to tetragonal phase, T (~400 K), then to rhombic phase, O (~300 K) and finally to rhombohedral phase, R (~210 K). Synthesis conditions [2, 3], piezoelectric [4–6] and dielectric investigations [7–12] of solid solution BTZx can be found in the literature.
The Ti and Zr ions have the similar configuration of external orbitals (Ti: 3s 23p 63d 24 s 2, Zr: 4s 24p 64d 25 s 2) and different radius of ion orbitals (Ti: 68 pm, Zr: 80 pm). This similarity results in regular changes of physical properties of modified materials for different distribution of Zr ions. Statistical distribution of B′ and B″ ions cause broadening of the PT temperature region. This fact can be of essential importance in practical applications. BT and BTZx are used as multilayer capacitor ceramics, piezoelectric transducers, PTRC thermistors, and chemical sensors [13–15]. Zirconium titanate (ZT) is used in varistor and tunable microwave filters and as the technical ceramic component [14, 16, 17].
There are two aims of this work. The first is to determine the influence of Zr substitution on the dielectric properties of BTZx. The second is to analyze the change of the character of the paraelectric–ferroelectric (PE–FE) PT (from sharp or DPT to relaxor or glass-type behaviour [18–23]) in this material.
The lack of the structural phase transition at T m for x ≥ 0.10 suggests that the cause of maximum of ε′ is the change of clusters system polarizability.
Experimental
The polycrystalline BTZx samples were obtained by the calcination method in two sintering processes at temperatures: ~1320 and ~1620 K.
The XRD data were collected by Philips X’Pert Pro MD powder diffractometer using Kα1 radiation from Cu anode. The configuration was standard Bragg–Brentano setup with Ge (111) monochromator at the incident beam. All measurements were performed with the 0.008° step size at 15°–90° scanning range and the 285 s of measurement time for each angle step. The full pattern fitting procedure for quantitative analysis and structural parameters determining was carried out using Philips X’Pert HighScore Plus (PW3212) Release 2.1 software.
The micro-structure of BTZx was investigated by means of a Philips SEM 525 M electron microscope at room temperature.
A sample of the diameter 10 mm and thickness 1.5 mm was covered by silver paint electrodes. The dielectric measurements were performed automatically (QUATRO KRIO 4.0 with LCR Agilent 4824A meter and BDS 1100 cryostat). The measurements were made during cooling at a rate of 2 K/min, within the frequency range of 20 Hz–1 MHz.
Results and discussion
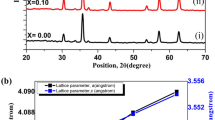
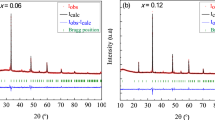
Figure 1 presents the X-ray pattern for polycrystalline sample of BTZ10 at the temperature of PE–FE phase transition (T m = 363 K). It shows that at this temperature only 13% of the tetragonal phase (a = b = 4.003 Å, c = 4.058 Å) and 87% of the cubic phase (a = 4.031 Å) exist.
This means that a 10% Zr concentration leads to freezing of the paraelectric, cubic structure. For BTZ30 only the cubic phase was observed at room temperature.
Figure 2 shows the SEM image of surface structure of pure BT (a) and BTZ30 (b, c) samples.
In this figure one can see crystallites with sharp edges of BT, the crystallites with smooth edges (b) and “micro-rollers” (c) of BTZ30.
Variety of crystallites (formed for high concentrations of Zr) follows from local gradients: of temperature and Zr ions concentration.
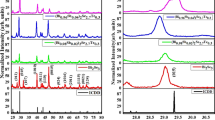
Figure 3 shows three clear maxima for the BT sample, involving the following structural phase transitions: C–T, T–O, O–R.
One can see a large increase of diffuseness of PT as well as a strong lowering of the T m with increase of concentration of zirconium (see Fig. 4).
Figure 5 present the temperature changes of the real part of the electric permittivity ε′(T) for BTZ20. The independence of the T m on the frequency of the applied electric field pointed on the DPT.
The following formula describes ferroelectric materials with DPT:
where ε m is the maximum value of the electric permittivity ε′, A, and γ are fixed constants.
In DPT the value of γ is close to 2. These values were compiled in Fig. 6, where:
Figure 3 shows a broad phase transition in BZT30. The PT is typical for relaxor materials.
From the glass model one can write the following Vogel-Fulcher equation [24, 25] involving T m,ν values for different frequencies:
where ν and ν 0 are the relaxation frequency at temperature T and a sufficiently high temperature, respectively, Ea is the activation energy of polar regions, k B is the Boltzmann constant and T f is the freezing temperature of polar regions.
Figure 7 present the dependence of (lnν) on (1000/(T m,ν−T f). From this graph one can calculate a glassy temperature value: T f ≈ 140 K.
Figures 8 and 9 show the value of phase angle as a function of temperature for concentrations x = 0.10 and 0.20. All Φ values are close to approx. −80 to −90° characteristic for the capacitive character of the material. This is also connected with the polar character of the material. Moreover, it is consistent with the occurrence of polar regions (clusters [26, 27]) in BTZx.
In PE–FE PT temperatures peaks of the phase angle were not observed. This suggests that the PT is not connected with structural PT but it follows from the change of the polar system in the region of PE–FE PT.
The change of system of these clusters, in the region of the PF phase transition, leads to formation of a strong maximum in measurements of the real part of the electric permittivity.
The clusters polarizability change with temperature is the source of dipolar polarization Pd [28–30]. This polarization originates from short-range interaction of electric dipoles. The growth of clusters size is a consequence of their long-range structural and electric collectivization.
Conclusions
The study presents the results of the dielectric measurements of BTZx. They confirm that a substitution of Zr ions in sublattice B leads to strong diffuseness and to lowering of the temperature of PE–FE phase transition. In the room temperatures, the BTZx (0 < x<0.20) is ferroelectric. For 0.10 < x<0.20 BTZ show DPT. For x ≥ 0.30 Zr ions lead to freezing of cubic structure below the temperature of the PE–FE phase transition. This behavior is similar to that of other relaxor type ferroelectrics.
The lack of structural phase transition at the temperature T m for 0.10 ≤ x ≤ 0.30, suggest that the PE–FE PT is connected solely with the change of clusters system polarizability.
The observed change of physical properties of BTZx can be applied in practice to change the work temperature of electromechanical devices.
References
Kajtoch C (1990) Doctor’s thesis Martin-Luther-University Halle-Wittenberg
Antonelli E, Silva RS, Hernandes AC (2006) Ferroelectrics 334(1):75
Bera J, Rout SK (2005) Mater Lett 59(1):135
Rehrig P et al (1999) J Appl Phys 86(3):1657
Yu Z et al (2002) J Appl Phys 92(3):1489
Yu Z et al (2000) Appl Phys Lett 77:1535
Dobal PS et al (2001) J Appl Phys 89(12):8085
Yu Z, Guo R, Bhalla AS (2000) J Appl Phys 88(1):410
Hennings D et al (2006) JACS 65(11):539
Weber U, Hennings D et al (2004) JACS 84(4):759
Hafer Ch, Mayer R, Boetger U, Waser R (2004) J Eur Ceram Soc 246:1473
Nanakorn N, Jalupoom P, Vaneesorn N, Thanaboonsombut A (2008) Ceram Int 34:779
Moulson AJ, Herbert JM (1990) Materials properties and applications. Chapman and Hall, London
Heywang W (1961) Solid State Electronics 3:51
Jonker GH (1964) Solid State Electronics 7:895
Fu LX, Zhang LY, Yao X (2008) J Electroceram 21(1–4):561
Ravez J, Simon A (2001) J Solid State Chem 162:260
Bokov AA, Ye Z-G (2000) Solid State Commun 116:105
Jonscher AK (1983) Dielectric relaxation in solids. Chelsea Dielectric Press Ltd, London
Cross LE (1994) Ferroelectrics 151:305
Kajtoch C (1997) Ferroelectrics 192:335
Ravez J, Simon A (2000) Physica Status Solidi (a) 178:793
Kajtoch C (2009) Ceram Int 35:2993
Vogel H (1921) Phys Z 22:645
Fulcher GS (1925) J Am Ceram Soc 8:339
Comes R, Lambert M, Guinier A (1970) Acta Crystall A26:244
Kajtoch C (1900) Ferroelectrics Lett 25:81
Burns G, Dacol FH (1982) Solid State Commun 42:9
Kajtoch C (1995) Ferroelectrics 172:465
Burns G, Dacol FH (1990) Ferroelectrics 104:25
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kajtoch, C. Influence of Zr-substitution on phase transitions character in polycrystalline Ba(Ti1−x Zr x )O3 . J Mater Sci 46, 1469–1473 (2011). https://doi.org/10.1007/s10853-010-4948-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4948-8