Abstract
The wettability of carbon (graphite and glassy carbon) by liquid aluminum was studied. A special molten salt (flux) system was developed under which perfect wettability (a zero contact angle) of liquid aluminum was achieved on carbon surfaces. The principal component of the flux is K2TiF6 dissolved in a molten alkali chloride. K2TiF6 is a multifunctional flux component as it performs the following tasks: (i) dissolves the oxide layer covering liquid aluminum, (ii) through an exchange reaction with liquid aluminum it ensures the necessary amount of Ti dissolved in liquid Al, which is needed to cover the Al/C interface by TiC. As TiC is a metallic carbide, it is perfectly wetted by liquid Al–Ti alloys. In this paper, the conditions of perfect wettability of carbon by liquid Al under MCl–K2TiF6 molten salts (fluxes) are found as function of: (i) the basic component of the flux (MCl = LiCl, or NaCl–KCl or CsCl), (ii) K2TiF6 content of the flux, (iii) temperature, (iv) flux:Al weight ratio, (v) specific surface area of Al, and (vi) specific surface area of carbon. A simplified theoretical equation is derived to reproduce the experimental data.







Similar content being viewed by others
Notes
From Table 2, the standard Gibbs energy change of reaction (3) is +833.7 ± 60 kJ at 1100 K, with an equilibrium constant of less than 10−36. Taking into account possible activities of the other components, the equilibrium vapor pressure of CF4 will be lower than 10−30 bar. This reaction takes place at the graphite/molten salt interface. The vapor pressure of 10−30 bar is not sufficient to form a new CF4 bubble. However, CF4 can enter the pores of the graphite. The closed system used in our experiments is about 10−3 m3 of total volume. Thus, if the law of ideal gas is supposed with the total volume occupied by the CF4 gas, the maximum number of moles of CF4 will be <10−36 mol. Thus, reaction (3) will lead to the formation of less than 10−36 mol of TiC, being much less than a single TiC molecule.
References
Li D, Li D, Zhang X, Sun T, Yao G (2010) J Alloys Comps 489:L1
Klinter AJ, Leon-Patino CA, Drew RAL (2010) Acta Mater 58:1350
Calderon NR, Voytovich R, Narciso J, Eustathopoulos N (2010) J Mater Sci 45:2150. doi:10.1007/s10853-009-3909-6
Barzilai S, Lomberg M, Aizenshtein M, Froumin N, Frage N (2010) J Mater Sci 45:2085. doi:10.1007/s10853-009-4018-2
Koltsov A, Crisci A, Hodaj F, Eustathopoulos N (2010) J Mater Sci 45:2062. doi:10.1007/s10853-009-4066-7
Drevet B, Pajani O, Eustathopoulos N (2010) Solar Energy Mater Solar Cells 94:425
Schmitz J, Brillo J, Egry I (2010) J Mater Sci 45:2144. doi:10.1007/s10853-010-4212-2
Kozlova O, Voytovich R, Protsenko P, Eustathopoulos N (2010) J Mater Sci 45:2099. doi:10.1007/s10853-009-3924-7
Budai I, Kaptay G (2010) J Mater Sci 45:2090. doi:10.1007/s10853-009-3907-8
Plevachuk Yu, Hoyer W, Kaban I, Köhler M, Novakovic R (2010) J Mater Sci 45:2051. doi:10.1007/s10853-009-4120-5
Klinter AJ, Leon CA, Drew RAL (2010) J Mater Sci 45:2174. doi:10.1007/s10853-009-4056-9
Kozlova O, Braccini M, Voytovich R, Eustathopoulos N, Martinetti P, Devismes M-F (2010) Acta Mater 58:1252
Israel R, Voytovich R, Protsenko P, Drevet B, Camel D, Eustathopoulos N (2010) J Mater Sci 45:2210. doi:10.1007/s10853-009-3889-6
Kaptay G, Bárczy T (2005) J Mater Sci 40:2531. doi:10.1007/s10853-005-1987-7
Kaptay G (2008) Comp Sci Technol 68:228
Verezub O, Kálazi Z, Buza G, Verezub NV, Kaptay G (2009) Surf Coat Technol 203:3049
Kaptay G (2006) Coll Surf A 282–283:387
Budai I, Kaptay G (2009) Metall Mater Trans A 40A:1524
Morita M, Baba H (1973) J Japan Inst Metals 37:315
Goddard DM, Burke PD, Kizer DE, Bacon R, Harrigan WC (1987) In: Engineered materials handbook, vol 1, Composites. ASM, USA, pp 867–873
Xia Z, Mao Z, Zhou Y (1991) Z Metallkund 82:766
Vidal-Setif MH, Lancin M, Marhic C, Valle R, Raviart JL, Daux JC, Rabinovits M (1999) Mater Sci Eng A272:321
Pippel E, Woltersdorf J, Doktor M, Blucher J, Degisher HP (2000) J Mater Sci 35:2279. doi:10.1023/A:1004787112162
Blucher JT, Narusawa U, Katsumata M, Nemeth A (2001) Composites A 32:1759
Etter T, Papakyriacou M, Schulz P, Uggowitzer PJ (2003) Carbon 41:1017
Kainer KU (2003) Metal matrix composites. Wiley-VCH, Weinheim, p 314
Blucher JT, Dobranszky J, Narusawa U (2004) Mater Sci Eng A387–389:867
Liu Z, Zhang G, Li H, Sun J, Ren M (2005) Mater Design 26:83
Rodriguez A, Sanchez SA, Narciso J, Louis E, Rodriguez-Reinoso F (2005) J Mater Sci 40:2519. doi:10.1007/s10853-005-1985-9
Wang TC, Fan TX, Zhang D, Zhang GD (2005) Mater Trans 46:1741
Rodriguez-Guerrero A, Sanchez SA, Narciso J, Louis E, Rodriguez-Reinoso F (2006) Acta Mater 54:1821
Flores-Zamora MI, Estrada-Guel I, Gonzalez-Hernandez J, Miki-Yoshida M, Amrtinez-Sanchez R (2007) J Alloy Comp 434–435:518
Tang Y, Huang Z, Liu Y, Liu L, Hu W (2008) Int J Mater Res 99:222
Orbulov IN, Németh A, Dobránszky J (2008) Mater Sci Forum 589:137
Tjong SC (2009) Carbon nanotube reinforced composites. Wiley-VCH, Berlin, p 228
Eustathopoulos N, Joud JC, Desre P (1974) J Mater Sci 9:1233. doi:10.1007/BF00551836
Naidich IV, Chuvashov IN, Ishuk NF, Krasovskii VP (1983) Poroshk Metal 6:67
Kaptay G (1991) Mater Sci Forum 77:315
Weirauch DA, Balaba WM, Perrotta AJ (1995) J Mater Res 10:640
Landry K, Kalogeropoulou S, Eustathopoulos N (1998) Mater Sci Eng 254:99
Eustathopoulos N, Nicholas MG, Drevet B (1999) Wettability at high temperatures. Pergamon, Amsterdam, p 420
Rocher JP, Quenisset JM, Naslan R (1989) J Mater Sci 24:2697. doi:10.1007/BF02385613
Kaptay G, Báder E, Bolyán L (2000) Mater Sci Forum 329–330:151
Nakae H, Yamamoto K, Sato K (1991) Mater Trans JIM 32:531
Rajan TPD, Pillai RM, Pai BC (1998) J Mater Sci 33:3491. doi:10.1023/A:1004674822751
Rams J, Ureña A, Escalera MD, Sánchez M (2007) Composites A 38:566
Ureña A, Rams J, Escalera MD, Sánchez M (2007) Composites A 38:1947
Masson B, Taghei MM (1989) Mater Trans JIM 30:411
Roy RR, Sahai Y (1997) Mater Trans JIM 38:995
Choh T, Kammel R, Oki T (1987) Z Metallkund 78:286
Kennedy AR, Karantzalis AE (1999) Mater Sci Eng A 264:122
Zhuxian Q, Mingjie Z, Yaxin Y, Zhenghan C, Grjotheim SK, Kvande H (1988) Aluminium 64:606
Zhuxian Q, Yaxin Y, Mingjie Z, Grjotheim SK, Kvande H (1988) Aluminium 64:1254
Lee MS, Terry BS, Grieveson P (1993) Metall Trans 24B:955
Jarfors A, Fredriksson H (1991) Mater Sci Eng A125:119
Svendsen L, Jarfors A (1993) Mater Sci Technol 9:948
Viala JC, Peillon N, Lochefert L, Bouix J (1995) Mater Sci Eng A203:222
Frage N, Frumin N, Levin L, Polak M, Dariel MP (1998) Metall Mater Trans 29A:1341
Jarfors AEW (1999) Mater Sci Technol 15:481
Kennedy AR, Weston DP, Jones MI (2001) Mater Sci Eng A316:32
Frumin N, Frage N, Polak M, Dariel MP (1997) Scripta Mater 37:1263
Contreras A, Leon CA, Drew RAL, Bedolla E (2003) Scripta Mater 48:1625
Contreras A, Bedolla E, Perez R (2004) Acta Mater 52:985
Lopez VH, Kennedy AR (2006) J Colloid Interface Sci 298:356
Tepliakov FK, Oskolskich AP, Kaluzhskii NA, Shusterov VS, Ivchenko VP (1991) Cvetnie Metall 9:54
Lee MS, Terry BS, Grieveson P (1993) Metall Trans 24B:947
El-Mahallawy N, Taha MA, Jarfors AEW, Fredriksson H (1999) J Alloy Comp 292:221
Murty BS, Kori SA, Chakraborty M (2002) Int Mater Rev 47:3
Birol Y (2006) J Alloys Comp 420:207
Birol Y (2008) J Alloys Comp 454:110
Baumli P, Kaptay G (2008) Mater Sci Eng A495:192
Smirnov MV (1973) Electrode potentials in molten chlorides. Nauka, Moscow, p 244 (in Russian)
Massalski TB (1990) Binary alloy phase diagrams, 2nd edn. ASM International, Materials Park, OH
Barin I (1993) Thermochemical properties of pure substances. VCh, New York
Chase MW (1985) J Phys Chem Data 14:731
Grjotheim K, Krohn C, Malinovsky C, Matiasovsky K, Thonstad J (1982) Aluminium electrolysis, 2nd edn. Aluminium-Verlag, Düsseldorf
Lee MS, Terry BS, Grieveson P (1994) Trans Inst Min Metall 103:C26
Chernov RV, Ermolenko IM (1973) Zh Neorg Himii 18:1372
Posipaiko VI, Alekseeva EA (1977) Phase diagram of molten salts, Part 2. Metallurgiia, Moscow (in Russian)
Chernov RV, Ermolenko NM (1970) Zh Neorg Himii 15:1962
Buhalova GA, Maslennikova GN, Rabkin DM (1962) Zh Neorg Himii 7:1640
Malcev VT, Buhalova GA (1965) Zh Neorg Himii 10:1464
Kobalev FV, Ioffe VM, Karcev VE (1970) Zh Neorg Himii 15:1211
Iida T, Guthrie RIL (1993) The physical properties of liquid metals. Clarendon Press, Oxford, p 288
Janz GJ, Dampier FW, Lakshminarayanan GR, Lorentz PK, Tomkins RPT (1968) Molten slats: volume 1, electrical conductance, density and viscosity data. NSRDS-NBS 15, Washington, USA, 139 pp
Danek V, Matiasovsky K (1989) Z Anorg Allg Chem 570:184
Martin-Garin L, Dinet A, Hicter JM (1979) J Mater Sci 14:2366. doi:10.1007/BF00737025
Roy RR, Ye J, Sahai Y (1997) Mater Trans JIM 38:566
Kaptay G (2004) Calphad 28:115
Esin IO, Bobrov NI, Petrushevskii MS, Geld PV (1974) Metalli 5:104
Kaufman L, Nesor H (1978) Calphad 2:325
Ansara I, Dinsdale AT, Rand MH (1998) Thermochemical database for light metal alloys, COST 507, vol 2. EUR18499 EN, Belgium
Keene BJ (1993) Int Mater Rev 38:157
Goumiri L, Joud JC (1982) Acta Metal 30:1397
Garcia-Cordovilla C, Louis E, Pamies A (1986) J Mater Sci 21:2787. doi:10.1007/BF00551490
Sarou-Kanian V, Millot F, Rifflet JFC (2003) Int J Thermophys 24:277
Kaptay G (2008) Mater Sci Eng A495:19
Janz GJ, Lakshminarayanan GR, Tomkins RPT, Wong J (1969) Molten slats: volume 2, section 2. Surface tension data, NSRDS-NBS 28, Washington, USA, pp 49–111
Silny A, Utigard TA (1996) J Chem Eng Data 41:1340
Ye J, Sahai Y (1996) Mater Trans JIM 37:1479
Roy RR, Sahai Y (1997) Mater Trans JIM 38:546
Roy RR, Utigard TA (1998) Metall Mater Trans 29B:821
Atkins PW (1990) Physical chemistry, 4th edn. University Press, Oxford
Kaptay G (2003) Interfacial forces, energies and phenomena. DSc thesis, Miskolc, Hungary
Emsley J (1989) The elements. Clarendon Press, Oxford
Lide DR (1993–1994) CRC handbook of chemistry and physics. CRC Press, Boca Raton
Kumar G, Pranbu KN (2007) Adv Colloid Interface Sci 133:61
Kaptay G (2005) Mater Sci Forum 473–474:1
Kosolapova TYa (1986) Properties, synthesis and application of refractory compounds. Metallurgiia, Moscow (in Russian)
Frisk K (2003) Calphad 27:367
Samsonov GV (1978) Physico-chemical properties of oxides. Metallurgiia, Moscow (in Russian)
Acknowledgements
This work was partly financed by the PhD program of the University of Miskolc and the Deák scholarship (P. Baumli) and partly by the NAP-NANO and by the CK 80255 OTKA-NKTH projects of Hungary.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: On the exchange reaction between the flux and liquid Al
When a NaCl–KCl–K2TiF6 salt comes into contact with liquid Al, the following exchange reaction is expected:
with round brackets indicating that the component is dissolved in the molten salt, while square brackets indicate that the given component is dissolved in the liquid metal. As follows from the binary Al–Ti phase diagram, a very low amount of Ti dissolved in liquid Al is sufficient to reach the two-phase region (Al–Ti) + Al3Ti [73]. Thus, Eq. 1 can be re-written in the following form, supposing that Al3Ti particles are dispersed in the liquid Al–Ti alloy:
The {} brackets indicate that the given pure phase is dispersed in the liquid metallic phase. Standard Gibbs energies of components are collected in Table 1 in the T-interval between 1000 and 1200 K. Most data are taken from [74]. For some components, data are missing from [74, 75] and other available sources of standard thermodynamic data. In these situations, data were estimated in this paper. In case of KAlF4 for this purpose the known values for K3AlF6, KF, AlF3 [74] and the information for the KF–AlF3 phase diagram [76] were used. In case of K2TiF6 for this purpose the measured equilibrium vapor pressure of TiF4 over K2TiF6 [77] combined with known thermodynamic data for KF and TiF4 [74, 75] and the KF–TiF4 phase diagram [78] were used.
The standard Gibbs energy change of reaction (2) is given in Table 2 in the same T-interval between 1000 and 1200 K. One can see that this value is very much negative (−870.0 ± 260 kJ at 1100 K), leading to very high value of the equilibrium constant of reaction (2): K = 9 × 1028 ··· 5 × 1053 at T = 1100 K. Such a high value of the equilibrium constant will guarantee that reaction (2) will be almost entirely shifted to the right hand side. In other words, all available Ti in the form of K2TiF6 will eventually appear in Al as dissolved Ti, or as part of the precipitated Al3Ti intermetallic phase (see also Appendix 7).
From Table 2, one can also see that the enthalpy change of reaction (2) is about −1387 ± 200 kJ, i.e., reaction (2) is very much exothermic. According to reaction (2), 6 mol of KF, 4 mol of AlF3, and 3 mol of Al3Ti participate in this reaction, with the total heat capacity of about 1160 ± 50 J/K [74, 75]. Thus, one can expect that the enthalpy change of reaction (2) will heat the system maximum by 1,200 ± 200 K. In reality, only a small fraction of this T-increase will be realized in the crucible due to a larger heat capacity of the whole system (NaCl–KCl, Al, crucible) and the natural heat loss. Nevertheless, the several hundreds of K of temperature increase reported in [65] for a similar reaction seems to be supported by this simplified calculation. Thus, one can expect that the real temperature within the crucible during the experiments will be somewhat higher than the nominal temperature indicated by the thermocouple positioned at some distance from the crucible. As a side effect, one might expect the undesired formation of aluminum carbide. However, characterization of the carbon/Al–Ti interfaces was not part of this project. It is a question remaining for following studies whether aluminum carbide is formed at the interface due to the above described exothermic effect.
Appendix 2: Liquidus temperature of the flux
From the economic and environmental points of view, the temperature of the process should be kept as low as possible. Due to the melting point of Al, the process should be run at least at 700 °C or above. Thus, the selected molten salt and the molten salt resulting from reaction (2) should have a liquidus temperature below 700 °C.
The binary equimolar NaCl–KCl system forms an azeotropic solution with a liquidus/solidus temperature of about 665 °C [79]. In this paper, the equimolar NaCl–KCl solution is denoted as (NaCl–KCl). The liquidus curve in the quasi-binary (NaCl–KCl)—K2TiF6 system was constructed by us (see Fig. 8) from the reciprocal Na+, K+//TiF6 2−, Cl− system reported in [80]. One can see that more than 90 wt% of K2TiF6 can be dissolved in (NaCl–KCl) without increasing the liquidus temperature above 700 °C.
Liquidus temperature in the quasi-binary (NaCl–KCl)–K2TiF6 system (drawn from the reciprocal Na+, K+//TiF6 2−, Cl− system of [80])
As it was shown above, K2TiF6 reacts with liquid Al forming K3AlF6 and KAlF4 according to reaction (2). The liquidus curve in the quasi-binary (NaCl–KCl)–K3AlF6 system was constructed by us (see Fig. 9) from the reciprocal NaCl–KCl–K3AlF6–Na3AlF6 system reported in [81, 82]. One can see that the liquidus temperature rises above 700 °C when more than 20 wt% of K3AlF6 is dissolved in (NaCl–KCl).
Unfortunately, no information on the (NaCl–KCl)–KAlF4 or on the (NaCl–KCl)–K3AlF6–KAlF4 systems was found by us in the literature. However, from the binary KF–AlF3 phase diagram it is quite obvious that when K3AlF6 is replaced by the same weight of KAlF4, the liquidus temperature will somewhat decrease. Thus, the liquidus curve in Fig. 9 can be considered as a maximum liquidus for the same wt% of (KAlF4 + K3AlF6) in the (NaCl–KCl) system. As mentioned above, a maximum of 20 wt% of (KAlF4 + K3AlF6) is allowed in the system to ensure that the liquidus temperature is kept below 700 °C. This corresponds to about 21 wt% maximum initial K2TiF6 content in the (NaCl–KCl) melt, in agreement with reaction (2).
In some experiments, NaF is added to (NaCl–KCl), as a less expensive substitute of K2TiF6 at least for the role to dissolve the oxide layer from the surface of liquid Al. The quasi-binary liquidus temperature for the (NaCl–KCl)–NaF system is shown in Fig. 10. It is constructed from ternary data of [83]. One can see that the liquidus temperature can be kept below 700 °C if the content of dissolved NaF does not reach 21 wt%.
Liquidus temperature in the quasi-binary (NaCl–KCl)–NaF system (drawn from the ternary NaCl–KCl–NaF system of [83])
Based on the above phase diagram information, the (NaCl–KCl) + 10 wt% K2TiF6 system will be used by us as a “standard” flux. In some experiments, a maximum of 10 wt% of NaF will be added. All these salts will have their liquidus temperature below 700 °C with and/or without their exchange reaction with liquid Al.
Appendix 3: Density of the flux
To ensure effective phase separation between liquid Al and the covering flux, the density of the molten salt system should be kept significantly below the density of liquid Al. Density values of some pure salts are collected in Table 3. One can see that all the possible components of the salts have somewhat lower densities compared to liquid Al. In the first approximation one can suppose that the density of (NaCl–KCl)-based fluxes are linear combinations of the densities of pure components multiplied by their weight ratios. This is quite well confirmed by actual measurements [87, 88].
Then, the density of the (NaCl–KCl) + 10 wt% K2TiF6 flux at 1100 K will be about 1576 kg/m3, which is lower by 32% compared to the density of liquid Al. According to reaction (2), the salt (NaCl–KCl) + 3.6 wt% K3AlF6 + 5.9 wt% KAlF4 will form from the (NaCl–KCl) + 10 wt% K2TiF6 flux. The approximate density of this salt is about 1542 kg/m3. Thus, in the course of reaction (2) the density of the salt is expected to decrease by about 2%, while the density of liquid Al is expected to increase slightly, due to alloying by heavier Ti.
Thus, we can conclude that the “standard” flux (NaCl–KCl) + 10 wt% K2TiF6 system with and/or without its exchange reaction with liquid Al will have a significantly lower density compared to liquid Al, i.e., the flux will form a stable liquid layer on the top of liquid Al.
Appendix 4: Chemical interaction between flux and graphite
In principle, TiC can be formed due to the chemical interaction of the K2TiF6 of the flux and graphite:
As one can see from Table 2, the standard Gibbs energy change of this reaction is a highly positive value, i.e., reaction (3) in reality does not take place.Footnote 1 Thus, the graphite surface cannot be covered by TiC using the molten salt alone. The spontaneous reducing effect of liquid aluminum is needed to reduce Ti from its Ti+4 form (see Appendix 1). Only the reduced form of Ti is able to form the TiC layer on graphite (see below), even if its activity is lowered due to alloy formation.
Appendix 5: Thermodynamic conditions to precipitate TiC at the Al–Ti/C interface
The next condition for our successful experiments is to meet thermodynamic criteria to form TiC at the Al–Ti/C interface. Without dissolved Ti in liquid Al, the following chemical interaction is expected to take place:
As follows from Table 2, reaction (4) will take place spontaneously at any reasonable temperature with liquid Al. When the content of dissolved Ti in liquid Al is sufficient to form Al3Ti, the following chemical reaction with graphite can also take place:
In principle, reactions (3–5) should be analyzed together. However, due to highly positive Gibbs energy change of reaction (3), in the first approximation only reactions (4–5) will be considered here. As follows from Table 2, the standard Gibbs energy of reaction (5) is less negative at 0 K compared to reaction (4). However, at T = 1100 K reaction (5) is accompanied with a more negative standard Gibbs energy change compared to reaction (4). The competition between reactions (4–5) is more obvious if one considers the following exchange reaction, obtained as the difference between reactions (4) and (5):
From the Gibbs energy values given in Table 2, one can see that this reaction is improbable at low temperatures, but becomes more and more probable at higher temperatures. At a given critical temperature T cr, the Gibbs energy of reaction (6) becomes zero. From the values given in Table 2, this takes place at T cr = 1026 ± 100 K = 753 ± 100 °C. The uncertainty of this value is quite high. Unfortunately, similar uncertainties can be found in the literature (see Table 4). As follows from Table 4, T cr is found between 693 and 900 °C in the literature [55–60].
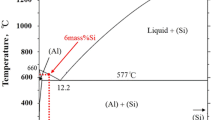
The above findings are super-positioned onto the binary Al–Ti phase diagram [73] in Fig. 11. According to the dotted lines of Fig. 11, there are two thermodynamic conditions to form a stable TiC phase at the Al–Ti/C interface:
The Al-corner of the binary Al–Ti phase diagram [73] with superimposed phase transition lines at the Al–Ti/C interface. Bold lines: equilibrium lines of the Al–Ti equilibrium phase diagram. The almost vertical dotted line is calculated by Eq. 9. The horizontal dotted line is drawn to higher Ti-contents from the point of intersection of the almost vertical dotted line with the liquidus line (the coordinates of this intersection point: 750 °C and 0.40 wt% Ti)
-
(i)
temperature should be above 750 °C,
-
(ii)
Ti-content of the remaining melt should be above a certain T-dependent value. The critical Ti-content is around C Ti,cr = 0.40 wt% at 750 °C. This Ti-content is the coordinate of the liquidus line at 750 °C. The critical Ti-content slightly increases with increasing T. The (almost) vertical dotted line in Fig. 11 was calculated using the T-dependence of the partial excess Gibbs energy of Ti in infinitely diluted solution of liquid Al (G is in kJ/mol, T is in K):
Equation 7 was obtained from fitting the liquidus in the Al-rich corner of the Al–Ti phase diagram [73] (see Fig. 11), coupled with the Gibbs energy of formation data of Al3Ti [74] (see Table 1). The mathematical form of Eq. 7 is in agreement with thermodynamic constraints [89]. Equation 7 is in a reasonable agreement with experimental heat of mixing data [90]. At temperatures around the liquidus, Equation 7 is also in a reasonable agreement with previous Calphad assessments [58, 91, 92]. The almost vertical dotted line in Fig. 11 corresponds to the following equilibrium:
In agreement with reaction (8), the equilibrium Ti-content can be calculated as:
This equation is derived by supposing that the solubility of carbon in the Al–Ti liquid alloy is zero and that activity of Al in the alloy is close to 1. The critical Ti-content calculated by Eq. 9 increases from 0.40 wt% at 750 °C to 0.42 wt% at 900 °C. Based on Fig. 11, the “standard” temperature of 800 °C is selected for our experiments. It is expected that at this T reaction (6) will be shifted to the right and the Al–Ti/C interface will be covered by TiC, supposing sufficient amount of Ti is added to the system through K2TiF6.
Appendix 6: The condition of perfect wettability
Now let us consider a liquid (L) metallic droplet on a solid surface (S) in the environment of a molten flux (F). The contact angle (Θ) of the droplet can be written by the Young equation applied to this case:
where σSF, σSL, and σLF are interfacial energies in solid/flux, solid/liquid metal, and liquid metal/flux systems, respectively. By definition, the adhesion energy in the solid/flux (W SF) and in the solid/liquid metal (W SL) can be written as:
where σSG, σLG, and σFG are surface energy of the solid and surface tension of the liquid metal and molten flux, respectively. Let us express σSF and σSL from Eqs. 11a–b and substitute these values into Eq. 10:
The condition of perfect wettability of S by L under F is that cos Θ = 1. Substituting this into Eq. 12, the critical W SL value ensuring perfect wettability is obtained as:
On the other hand, adhesion energy in the S/L system can be modeled as a linear combination of adhesion energies along a heterogeneous solid/liquid interface. In the first approximation, let us suppose that some βTiC fraction of the initial graphite (C) surface is covered by TiC. Then, the average adhesion energy is written as:
where W C/L and W TiC/L are the adhesion energies in graphite/liquid metal and TiC/liquid metal systems. As follows from Eq. 14, at βTiC = 0: \( W_{\text{SL}} = W_{\text{C/L}} \), while at βTiC = 1: \( W_{\text{SL}} = W_{\text{TiC/L}} \). Let us substitute Eq. 14 into Eq. 13 and let us express the critical value of βTiC,cr above which the contact angle becomes zero:
Perfect wettability of graphite by liquid Al under the molten flux can be achieved only if the value of βTi,cr ≤ 1, as unity is the physical limit of surface coverage. In other words, perfect wettability will be achieved if βTi ≥ βTi,cr. Now, let us estimate the value of βTi,cr using Eq. 15. Let us perform this analysis supposing that Eq. 2 has been shifted to the right, and, as a result, sufficient Ti is dissolved in liquid Al to ensure the formation of at least some TiC, in accordance with Fig. 11. Thus, we consider the following composition of solution phases: L = Al–Ti alloy, F = (NaCl–KCl)–KAlF4–K3AlF6 flux. The calculations will be performed for the “standard” temperature of 800 °C (see Appendix 5).
Surface tension of different pure molten salts is given in Table 5. One can see that in the F = (NaCl–KCl)–KAlF4–K3AlF6 flux, the potassium salts are surface active. The estimated surface tension value is σFG = 90 ± 5 mJ/m2.
The contact angle of Na3AlF6 on a graphite surface is about 140° [76]. Combining this value with its surface tension (Table 5), and the Young equation, a value of solid/liquid interfacial energy of 272 mJ/m2 is obtained (the solid/gas interfacial energy of graphite is taken as 150 mJ/m2 [71]). From the contact angle of NaCl on C (113° [71]), the solid/liquid interfacial energy equals 195 mJ/m2. Thus, among sodium salts, the chlorides are interface active at the flux/graphite interface. Let us suppose that this is also the case for the potassium salts. Then, KCl will be segregated to the flux/graphite interface with an adhesion energy of W SF = 120 ± 5 mJ/m2 [71].
As follows from Table 5, Ti is not surface active in liquid Al [93]. Thus, the following value is used here: σLG = 1023 ± 30 mJ/m2. This value corresponds to the nonoxidized surface of aluminum [38, 94–97].
The interfacial energy in the (NaCl–KCl)/Al system with different additives was studied in [87, 99–102]. Using the experimental data and theories given in these papers, the estimated value for our system is found as: σLF = 600 ± 30 mJ/m2.
The adhesion energy in the nonreactive M/C system (M: liquid metal, C: covalent element) can be estimated from the London-van-der-Waals dispersion interaction [103, 104]. Taking into account also the repulsive term using the Lennard-Jones potential and dividing the resulting interaction energy by the average molar interface area, the adhesion energy is obtained with a negative sign as:
with f = 1.0, geometrical factor [97], V i is the molar volume of component i (m3/mol), N Av is the Avogadro number (6.02 × 1023 L/mol), αi is the polarizability of the gaseous atom i (m3), R i is radius of atom i (m), I i is the first ionization energy of gaseous atom i (J/mol). The physical constants for carbon: V C = 5.3 × 10−6 m3/mol [105], R C = 1.85 × 10−10 m [105], I C = 1.08 × 106 J/mol [105], and αC = 1.76 × 10−30 m3 [106]. The physical constants for Al (and Ti): V M = 1.1 × 10−5 (1.2 × 10−5) m3/mol [84], R M = 1.43 × 10−10 (1.45 × 10−10) m [105], I M = 5.77 × 105 (6.58 × 105) J/mol [105], αM = 6.8 × 10−30 (14.6 × 10−30) m3 [106]. Substituting these values into Eq. 16: W C/Al = 78 ± 15 mJ/m2, W C/Ti = 170 ± 34 mJ/m2. Using the Young-Dupre equation and Table 5, the contact angles are obtained as 157° and 154° in the Al/C and Ti/C systems, respectively. These values fit well into the data-bank on contact angles of nonreactive covalent/metallic couples [36, 37, 107]. From the definition of adhesion energy, the solid/liquid interfacial energies can be calculated (using σCG = 150 mJ/m2 [71] and σLG values from Table 5) being equal to 1095 mJ/m2 for the Al/C and to 1744 mJ/m2 for the Ti/C interface. Thus, Ti in the Al–Ti alloy is not interface active at the C/L interface (in the absence of chemical interaction). Therefore, the W C/Al value is selected for W C/L = 78 ± 15 mJ/m2.
Now, let us estimate the interfacial energies in the Al/TiC and Ti/TiC systems using the following equation [108]:
where f b,M is the bulk packing fraction of the solid metal M, Δm H i is melting enthalpy of component i (J/mol), Ω i–j is the interaction energy between components i and j of the liquid alloy in the approximation of the regular solution model (J/mol). Values of the general parameters: Δm H Ti = 2.09 × 104 J/mol [105], Δm H C = 1.05 × 105 J/mol [105], \( \Updelta_{\text{f}} H_{\text{TiC}}^{\text{o}} \) = −1.72 × 105 J/mol [74], V TiC = 1.4 × 10−5 m3/mol [109]. The parameter values for M = Al: f b = 0.74, V Al = 1.1 × 10−5 m3/mol [84], Ω Al–Ti = −6.6 × 104 J/mol (see Eq. 7), Ω Al–C = −1.6 × 104 J/mol [58]. Then, the calculated value from Eq. 17: σTiC/Al = 1020 ± 200 mJ/m2. The parameter values for M = Ti: f b = 0.74, V Ti = 1.2 × 10−5 m3/mol [84], Ω Ti–Ti = 0 (by definition), Ω Ti–C = −1.8 × 105 J/mol [110]. Then, the calculated value from Eq. 17: σTiC/Ti = 534 ± 110 mJ/m2. As σTiC/Ti < σTiC/Al, Ti is an interface active component at the Al–Ti/TiC interface. Nevertheless, Ti is not able to decrease the interfacial energy of the Al–Ti/TiC interface significantly, due to its low solubility (see Fig. 11) and negative excess Gibbs energy (see Eq. 7). Thus, the requested value approximately equals: σTiC/L = 1000 ± 210 mJ/m2.
The adhesion energy W TiC/L can be calculated from its definition of Eq. 11b: \( W_{\text{TiC/L}} = \sigma_{\text{TiC/G}} + \sigma_{\text{L/G}} - \sigma_{\text{TiC/L}} \). The surface energy of TiC is taken from [108]: σTiC/G = 2250 ± 250 mJ/m2. The surface tension of the liquid alloy was found above: σLG = 1023 ± 30 mJ/m2. The interfacial energy was estimated above: σTiC/Al = 1000 ± 210 mJ/m2. Then, the following result is obtained for the adhesion energy: WTiC/L = 2273 ± 490 mJ/m2.
Finally, the critical TiC coverage of the C-surface ensuring perfect wettability is found from Eq. 15 as: βTiC,cr = 0.72 ± 0.25. Thus, the possible interval of values of βTiC,cr is between 0.47 … 0.97. Hence, one can expect that liquid Al will perfectly wet carbon under the given molten flux if about 72% of the carbon surface is covered by TiC. This theoretical result is in agreement with experimental observation of [64] that liquid Al perfectly wets pure TiC under a fluoride flux.
Appendix 7: Mass balance requirements
Now, let us write the mass balance of Ti as follows:
where the masses of Ti (m Ti, kg) mentioned in Eq. 18 correspond to the following items:
-
(i)
the mass introduced into the system by the K2TiF6-content of the flux (in),
-
(ii)
the mass dissolved in liquid Al (Al),
-
(iii)
the mass bonded into TiC at the Al–Ti/C interface (TiC),
-
(iv)
the mass spent to dissolve the oxide layer from the surface of the Al droplet (ox),
-
(v)
the mass lost due to evaporation, etc. (loss).
Now, let us model shortly each item of Eq. 18. For this, let us consider the initial mass of the flux (m F, kg), the initial mass of liquid Al (m Al, kg), the initial surface area of carbon (A C, m2). The task is to make the full surface A C wettable by liquid Al. The goal of this model is to find the critical condition of perfect wettability. Thus, all conditions will be taken at minimum values ensuring perfect wettability.
Let us denote the initial concentration of K2TiF6 in the salt by \( C_{{\rm K}_{2}{\rm TiF}_{6}} \) (wt%). Then, the mass of Ti introduced into the system by the K2TiF6-content of the flux is written as:
where 2.0 × 10−3 is the ratio of molar masses of Ti to K2TiF6, divided by 100.
According to Fig. 11, about 0.406 wt% of Ti dissolved in liquid Al is needed to ensure stability of the TiC at the Al–Ti/C interface at 800 °C. Thus, the following equation describes the minimum required mass of Ti dissolved in liquid Al:
If intermetallic particles Al3Ti precipitate from liquid Al, the actual mass of Ti will be higher than that calculated by Eq. 20.
According to nanothermodynamics, there is a minimum thickness of phases above which they behave in a “macroscopic” way. Let us take this thickness of the TiC layer being equal to δTiC,cr = 10 nm = 10−8 m. Let us suppose that this is the minimum thickness of the TiC layer, which is able to provide perfect wettability of C by Al–Ti. Then, the minimum required volume of TiC is calculated as: \( V_{\text{TiC}} = A_{\text{C}} \cdot \delta_{\text{TiC,cr}} \cdot \beta_{\text{TiC,cr}} \). Multiplying this volume by the density of TiC (4280 kg/m3 [109]) and by the ratio of molar masses of Ti to TiC (0.80), the minimum mass of Ti, required in the TiC-bonded form to ensure perfect wettability is obtained as:
In Eq. 21, βTiC,cr = 0.72 and δTiC,cr = 10−8 m are involved. In reality, the thickness of the TiC layer might be above 10 nm, or the coverage of the carbon surface can also be higher than the critical coverage. In both cases, the actual mass of Ti will be higher than that calculated by Eq. 21. Comparing Eqs. 20 and 21, it is obvious that the amounts of Ti dissolved in liquid Al and that bonded in TiC become equal if 168 m2 of carbon is in contact with 1 kg of liquid Al.
Let us suppose that Al is used as ingots of side lengths a * a * b with b = 3 * a. Then, taking into account the density of Al (2700 kg/m3 [105]), the surface area of Al is calculated as: \( A_{\text{Al}} = 0.035 \cdot m_{\text{Al}}^{2/3} \). Let us suppose that this surface area is covered by a thin oxide layer of thickness δox. Then, the total mass of Al2O3 introduced into the system in the form of an oxide layer is calculated as (taking into account the density of Al2O3 of 3900 kg/m3 [111]): \( m_{\text{ox}} = 137 \cdot \delta_{\text{ox}} \cdot m_{\text{Al}}^{2/3} \). As experiments show [49], chloride salts such as (NaCl–KCl) do not dissolve alumina. However, the solubility of alumina increases with increasing the concentration of fluorides. For simplicity, let us suppose that the solubility of alumina in the flux (C sol) is proportional to the concentration of K2TiF6 in the flux: \( C_{\text{sol}} = 0.01 \cdot C^{*} \cdot C_{{{\text{K}}_{ 2} {\text{TiF}}_{ 6} }} \) (with C* being the solubility of alumina in pure K2TiF6). By definition, the solubility of alumina in the flux is expressed as: \( C_{\text{sol}} \equiv 100 \cdot m_{\text{ox}} /m_{\text{F}} \). From here, the amount of flux being able to dissolve the oxide can be written: \( m_{\text{F}}^{\text{ox}} = 100 \cdot m_{\text{ox}} /C_{\text{sol}} \). Substituting into this equation, the above equations for m ox and C sol, the mass of flux needed to dissolve the oxide layer is found. From here, the mass of Ti within this flux can be calculated by an equation being analogous to Eq. 19: \( m_{\text{Ti}}^{\text{ox}} = 2740 \cdot {\frac{{\delta_{\text{ox}} }}{{C^{*} }}} \cdot m_{\text{Al}}^{2/3} \). For simplicity we can write:
The thickness of the fresh oxide layer is about δox = 100 nm. The value of C* depends on temperature and composition of the salt (for a fixed equimolar (NaCl–KCl) salt it is constant at given T). In the first approximation, C* = 0.1 wt%. Substituting these two approximated values into the above equation: \( k_{\text{ox}} \equiv 2740 \cdot {\frac{{\delta_{\text{ox}} }}{{C^{*} }}} \cong 2.7 \times 10^{ - 3} \).
The loss of Ti is mainly due to its dissociation with the consequent evaporation of TiF4 and oxidation by the surrounding gas. Under the given conditions, the mass of lost Ti is proportional to the concentration of K2TiF6 in the flux:
The semi-empirical coefficient k loss is a function of temperature, time, and other experimental conditions.
Now, let us substitute Eqs. 19–23 into Eq. 18:
From Eq. 24, the critical mass ratio of the flux to Al to ensure perfect wettability can be found as:
One can see that there are parameters with uncertain values (k ox, k loss) in Eq. 25. That is why an experimental program was performed to find the values of these semi-empirical parameters. The critical condition of perfect wettability of graphite by liquid Al under NaCl–KCl–K2TiF6 flux was measured and the results were compared to Eq. 25 in the main text of this paper (see Figs. 2–7).
When all parameters are fixed except the K2TiF6 concentration, Eq. 25 can be written in terms of the critical ratio (m F/m Al)cr needed for perfect wettability as:
Thus, plotting the experimentally determined values of (m F/m Al)cr as function of \( 1/C_{{{\text{K}}_{ 2} {\text{TiF}}_{ 6} }} \), the semi-empirical parameters A and B can be found by a linear fit in accordance with Eq. 25a. Using Eqs. 25b and c, the values of k loss and k ox can be evaluated if m Al and A C are known.
Rights and permissions
About this article
Cite this article
Baumli, P., Sytchev, J. & Kaptay, G. Perfect wettability of carbon by liquid aluminum achieved by a multifunctional flux. J Mater Sci 45, 5177–5190 (2010). https://doi.org/10.1007/s10853-010-4555-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4555-8