Abstract
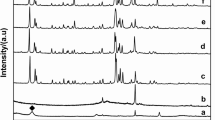
The influence of basicity and anionic composition (CrO 2−4 and Cl−) was studied in the synthesis of chromate-cancrinite and chlorosodalite zeolites. Zeolite X was used as starting material by using different NaOH concentrations (3, 8 and 16 M) and variable weight ratio (NaCl/(NaCl + K2CrO4)) equal to: 0.00, 0.25, 0.50, 0.75, and 1.00 in such a way that the total grams were 1.5 g. The syntheses were carried out at 80 °C and autogenous pressure during 40 h. The crystals obtained were characterized by powder X-ray diffraction (XRD), FT-IR spectroscopy, and scanning electron microscopy (SEM) coupled with an energy-disperse X-ray detector (EDX). Depending on the basicity employed and anionic composition (CrO 2−4 and Cl−), the chromate-cancrinite or chlorosodalite zeolite was obtained. Owing to a low 3 M NaOH concentration and 100% Cl−, chlorosodalite mixed with zeolite X was observed in the XRD pattern. Owing to another Cl−–CrO 2−4 proportions, inclusive to 100% CrO 24 , the starting material were not transformed (3 M NaOH). Pure chromate-cancrinite was produced when 8 and 16 M NaOH and 0% Cl− concentrations were used. Chloride anions showed a strong structure directing effect for sodalite framework structure in comparison with chromate anions which favoring the cancrinite structure. Therefore, we determined pure chlorosodalite, when it was used until 75%Cl− (8 M NaOH) and 25% (16 M NaOH). Results showed that cancrinite or sodalite zeolites are separately formed.







Similar content being viewed by others
References
Barrer RM, Cole JF, Villiger H (1970) J Chem Soc (A) 1523
Weller MT (2000) J Chem Soc, Dalton Trans 4227
Hermeler G, Buhl J-Ch, Hoffman W (1991) Catal Today 8:415
Pettine M, Campanella L, Sillero F (2002) Environ Sci Technol 36:901
Linares CF, Madriz S, Goldwasser MR, Urbina de Navarro C (2001) Stud Surf Sci Catal 135:331
Cundy C, Cox P (2005) Microporous Mesoporous Mater 82:1
Lin DC, Xu X-W, Zuo F, Long Y-C (2004) Microporous Mesoporous Mater 70:63
Flaningen EM, Khatami H, Szymanski H (1971) Adv Chem Ser: Mol Sieve Zeolite 101:201
Farmer VC (1974) The infrared spectra of minerals. Mineralogical Society, London, Monograph 4
Hackbarth K, Gesing ThM, Fechtelkord M, Stief F, Buhl J-Ch (1999) Microporous Mesoporous Mater 30:347
Haggerty GM, Bowman RS (1994) Environ Sci Technol 28:452
Acknowledgements
Authors are grateful to FONACIT trought F-2001000774, F-2001001442 and CDCH-UC for funding the research carried out in this work and Profs. Manuela Sánchez and Jeff Wilkesman for technical assistance. The unknown referee and the editor are thanked for their helpful advices.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ocanto, F., Álvarez, R., Moy, B. et al. Study of the influence of the basicity and chromate-chloride anionic composition in the synthesis of the cancrinite–sodalite system. J Mater Sci 43, 190–196 (2008). https://doi.org/10.1007/s10853-007-2083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2083-y