Abstract
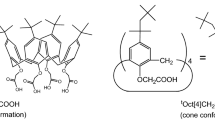
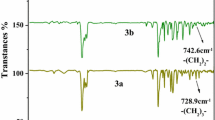
Trialkyl-monoacetic acid derivatives of p–t-octylcalix[4]arene and calix[4]arene were prepared to investigate the effect of the alkyl branches attached to the phenoxy oxygen atoms and the p-position on the selective extraction of Li+ over Na+. Alkyl branches on the phenoxy oxygen atoms remarkably affected the Li+ selectivity, whereas those at the p-position had less effect. The former can contribute to excluding Na+ extraction while enabling Li+ extraction. Optimal selection of the alkyl branch improves the Li+ selectivity of calix[4]arene. However, sterically-hindered p–t-octylcalix[4]arene with three 2-ethylbutyl branches exhibited opposite selectivity.









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Gruber, P.W., Medina, P.A., Keoleian, G.A., Kesler, S.E., Everson, M.P., Wallington, T.J.: Global lithium availability, a constraint for electric vehicles? J. Ind. Ecol. 15(5), 760–775 (2011)
Liu, G., Zhao, Z., Ghahreman, A.: Novel approaches for lithium extraction from salt-lake brines: a review. Hydrometallurgy 187, 81–100 (2019)
Kesler, S.E., Gruber, P.W., Medina, P.A., Keoleian, G.A., Everson, M.P., Wallington, T.J.: Global lithium resources: relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 48, 55–69 (2012)
Kaunda, R.B.: Potential environmental impacts of lithium mining. J. Energy Nat. 38(3), 237–244 (2020)
Reck, B.K., Graedel, T.E.: Challenges in metal recycling. Science 337(6095), 690–695 (2012)
Martin, G., Rentsch, L., Höck, M., Bertau, M.: Lithium market research—global supply, future demand and price development. Energy Storage Mater. 6, 171–179 (2017)
Swain, B.: Separation and purification of lithium by solvent extraction and supported liquid membrane, analysis of their mechanism: a review. J. Chem. Technol. Biotechnol. 91(10), 2549–2562 (2016)
Pedersen, C.J.: Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89(10), 2495–2496 (1967)
Frensdorff, H.K.: Stability constants of cyclic polyether complexes with univalent cations. J. Am. Chem. Soc. 93(3), 600–606 (1971)
Izatt, R.M., Pawlak, K., Bradshaw, J.S., Bruening, R.L.: Thermodynamic and kinetic data for macrocycle interactions with cations and anions. Chem. Rev. 91(8), 1721–2085 (1991)
Glue, K. (ed.): Macrocyclic chemistry—current trends and future perspectives. Springer, Netherlands (2005)
Bianchi, A., Bowman-James, K., García-España, E. (eds.): Supramolecular chemistry of anions. Wiley-VCH, New York (1997)
Katsuta, S., Imoto, T., Kudo, Y., Takeda, Y.: Selective extraction of lithium with a macrocyclic trinuclear complex of (1,3,5-trimethylbenzene)ruthenium(II) bridged by 2,3-dioxopyridine. Anal. Sci. 24(10), 1215–1217 (2008)
Katsuta, S., Nomura, H., Egashira, T., Kanaya, N., Kudo, Y.: Highly lithium-ion selective ionophores: macrocyclic trinuclear complexes of methoxy-substituted arene ruthenium bridged by 2,3-pyridinediolate. New J. Chem. 37(11), 3634–3639 (2013)
Gutsche, C.D., Muthukrishnan, R.: Calixarenes. 1. Analysis of the product mixtures produced by the base-catalyzed condensation of formaldehyde with para-substituted phenols. J. Org. Chem. 43(25), 4905–4906 (1978)
Gutsche, C.D. (ed.): Calixarenes revisited. The Royal Society of Chemistry, Cambridge (1998)
Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J. (eds.): Calixarenes 2001. Kluwer Academic Publishers, Netherlands (2001)
Lumetta, G.J., Rogers, R.D., Gopalan, A.S.: Calixarenes for separations. American Chemical Society, Washington, DC (2000)
Ohto, K.: Review of the extraction of metal cations with calixarene derivatives. Solvent Extr. Res., Dev. Jpn. 17, 1–18 (2010)
Ohto, K.: Review on adsorbents incorporating calixarene derivatives for metals recovery and hazardous ions removal—concept of adsorbent design and classification of adsorbents. J. Incl. Phenom. Macrocycl. Chem. 101(3), 175–194 (2021)
Ohto, K.: Chap. 3 Molecular design and metal extraction behavior of calixarene compounds as host extractants. Ion Exch. Solv. Extr. 21, 81–127 (2013)
Ohto, K., Furugou, H., Morisada, S., Kawakita, H., Isono, K., Inoue, K.: Stepwise recovery of molybdenum, tungsten, and vanadium by stripping using an amino-type “Trident” molecule. Solvent Extr. Ion Exch. 39, 512–532 (2021)
Ohto, K.: Research on various structural effects of calix[4]arene derivatives on extractive separation behavior of metal ions. J. Ion Exch. 30(2), 17–28 (2019)
Sadamatsu, H., Morisada, S., Kawakita, H., Ohto, K.: Allosteric coextraction of sodium and metal ions with calix[4]arene derivatives 3. Effect of propyl groups on size-discrimination for the second coextracted ion. Solvent Extr. Ion Exch. 33(3), 264–277 (2015)
Sadamatsu, H., Hanada, T., Morisada, S., Kawakita, H., Ohto, K.: Comprehensive comparison of alkali metal extraction with a series of calix[4]arene derivatives with propyl and/or acetic acid groups. J. Incl. Phenom. Macrocycl. Chem. 84, 87–97 (2016)
Ohto, K., Yano, M., Inoue, K., Yamamoto, T., Goto, M., Nakashio, F., Shinkai, S., Nagasaki, T.: Solvent extraction of trivalent rare earth metal ions with carboxylate derivatives of calixarenes. Anal. Sci. 11(6), 893–902 (1995)
Gutsche, C.D., Levine, J.A.: Calixarenes. 6. Synthesis of a functionalizable calix[4]arene in a conformationally rigid cone conformation. J. Am. Chem. Soc. 104(9), 2652–2653 (1982)
Ohto, K., Fuchiwaki, N., Morisada, S., Kawakita, H., Weigand, J.J., Marco, W.: Comparative extraction of aluminum group metals using acetic acid derivatives with three different-sized frameworks for coordination. Separations 8(11), 211 (2021)
Ohto, K.: unpublished data, in CDCl3 at 20 °C (1995)
Acknowledgements
This work was financially supported in part by the Sasakawa Scientific Research Grant No. 25-301 from “The Japan Science Society” and by JSPS KAKENHI Grant Number (Grant-in-Aid for “Scientific Research (C) 25410192) from JSPS”. The NMR measurements were conducted at Analytical Research Center for Experimental Sciences, Saga University supported by “Project for Promoting Public Utilization of Advanced Research Infrastructure”. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was funded by Japan Society for the Promotion of Science, KAKENHI (C) 25410192, Sasakawa Scientific Research Grant, 25-301.
Author information
Authors and Affiliations
Contributions
KO planned this research work, elucidated the extraction mechanism, and wrote the paper. HS prepared most of the reagents, measured spectra data, and carried out extraction. TH prepared one of the reagents, measured spectra data, and carried out extraction. SM discussed with spectra data of the reagents. HK supported for the extraction reaction and for drawing the theoretical lines to estimate the extraction constants and separation factors.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ohto, K., Sadamatsu, H., Hanada, T. et al. Li-selective calix[4]arene with trialkyl-monoacetic acid groups: effect of three alkyl branches and t-octyl groups at p-position on selectivity for Li extraction. J Incl Phenom Macrocycl Chem (2024). https://doi.org/10.1007/s10847-024-01233-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10847-024-01233-5