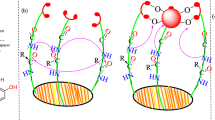
Abstract
Chelation and solution thermodynamic stability of a tripodal hydroxypyranone-based chelator (tris[(5-hydroxy-4-oxo-pyran-2-yl)methyl]benzene-1,3,5-tricarboxylate), TBHPY, towards biologically relevant divalent metal ions:Cu(II), Fe(II), Ni(II), Co(II) and Zn(II) were studied by potentiometric and spectroscopic methods in 9:1 (H2O:DMSO) medium. The metal ions formed ML, MLH2, MLH, MLH-2, and MLH-1 type complexes with high formation constants. The ligand was explored for its application as a potential fluorimetric sensor and examined in the presence of various cations. Nearly twofold quenching was observed upon addition of Gd(III) to TBHPY. The experimental, spectroscopic and thermodynamic stability results were validated with the theoretical quantum mechanical calculations using density functional theory (DFT). The geometrical structures, electronic properties,and bonding behavior of the complexes are described in detail.








Similar content being viewed by others
References
He, M., Fan, M., Peng, Z., Wang, G.: An overview of hydroxypyranone and hydroxypyridinone as privileged scaffolds for novel drug discovery. Eur. J. Med. Chem. 221, 113546 (2021). https://doi.org/10.1016/j.ejmech.2021.113546
Jiang, X., Zhou, T., Bai, R., Xie, Y.: Hydroxypyridinone-based iron chelators with broad-ranging biological activities. J. Med. Chem. 63, 14470–14501 (2020). https://doi.org/10.1021/acs.jmedchem.0c01480
Burgess, J., Rangel, M.: Hydroxypyranones, hydroxypyridinones, and their complexes. In: Advances in Inorganic Chemistry, pp. 167–243. Elsevier, Amsterdam (2008)
Saeedi, M., Eslamifar, M., Khezri, K.: Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 110, 582–593 (2019). https://doi.org/10.1016/j.biopha.2018.12.006
Baláž, Š, Uher, M., Brtko, J., Veverka, M., Bransová, J., Dobias, J., Pódová, M., Buchvald, J.: Relationship between antifungal activity and hydrophobicity of kojic acid derivatives. Folia Microbiol. 38, 387–391 (1993). https://doi.org/10.1007/BF02898762
Bransová, J., Brtko, J., Uher, M., Novotný, L.: Antileukemic activity of 4-pyranone derivatives. Int. J. Biochem. Cell Biol. 27, 701–706 (1995). https://doi.org/10.1016/1357-2725(95)00031-J
Loomis, L.D., Raymond, K.N.: Solution equilibria of enterobactin and metal-enterobactin complexes. Inorg. Chem. 30, 906–911 (1991). https://doi.org/10.1021/ic00005a008
Raymond, K.N., Cass, M.E., Evans, S.L.: Metal sequestering agents in bioinorganic chemistry: enterobactin mediated iron transport in E. coli and biomimetic applications. Pure Appl. Chem. 59, 771–778 (1987). https://doi.org/10.1351/pac198759060771
Rodgers, S.J., Lee, C.W., Ng, C.Y., Raymond, K.N.: Ferric ion sequestering agents. 15. Synthesis, solution chemistry, and electrochemistry of a new cationic analog of enterobactin. Inorg. Chem. 26, 1622–1625 (1987). https://doi.org/10.1021/ic00257a030
Hou, Z., Stack, T.D.P., Sunderland, C.J., Raymond, K.N.: Enhanced iron(III) chelation through ligand predisposition: syntheses, structures and stability of tris-catecholate enterobactin analogs. Inorg. Chim. Acta 263, 341–355 (1997). https://doi.org/10.1016/S0020-1693(97)05664-8
Tor, Y., Libman, J., Shanzer, A., Felder, C.E., Lifson, S.: Chiral siderophore analogs: enterobactin. J. Am. Chem. Soc. 114, 6661–6671 (1992). https://doi.org/10.1021/ja00043a008
Garrett, T.M., McMurry, T.J., Hosseini, M.W., Reyes, Z.E., Hahn, F.E., Raymond, K.N.: Ferric ion sequestering agents. 22. Synthesis and characterization of macrobicyclic iron(III) sequestering agents. J. Am. Chem. Soc. 113, 2965–2977 (1991). https://doi.org/10.1021/ja00008a027
Thakur, M., Baral, M., Kanungo, B.K.: Experimental and theoretical studies on coordination of a flexible cyclohexane based C3-symmetric tripodal ligand with iron, aluminum and gallium. Polyhedron 229, 116191 (2023). https://doi.org/10.1016/j.poly.2022.116191
Ng, C.Y., Rodgers, S.J., Raymond, K.N.: Ferric ion sequestering agents. 21. Synthesis and spectrophotometric and potentiometric evaluation of trihydroxamate analogs of ferrichrome. Inorg. Chem. 28, 2062–2066 (1989). https://doi.org/10.1021/ic00310a011
Thakur, M., Baral, M., Kanungo, B.K.: Design, synthesis, spectroscopic, photophysical and computational studies of a C3-symmetric hydroxyquinoline based tripod: TREN2OX and its interaction with Fe(III) and Al(III). J. Mol. Struct. 1248, 131436 (2022). https://doi.org/10.1016/j.molstruc.2021.131436
Sharma, S., Baral, M., Dash, D., Kanungo, B.K.: Synthesis, solution studies and DFT investigation of a tripodal ligand with 3-hydroxypyran-4-one scaffold. J. Incl. Phenom. Macrocycl. Chem. 101, 275–289 (2021). https://doi.org/10.1007/s10847-021-01088-0
Harrington, J.M., Chittamuru, S., Dhungana, S., Jacobs, H.K., Gopalan, A.S., Crumbliss, A.L.: Synthesis and Iron sequestration equilibria of novel exocyclic 3-hydroxy-2-pyridinone donor group siderophore mimics. Inorg. Chem. 49, 8208–8221 (2010). https://doi.org/10.1021/ic902595c
Xu, J., O’Sullivan, B., Raymond, K.N.: Hexadentate hydroxypyridonate iron chelators based on TREN-Me-3,2-HOPO: variation of cap size 1. Inorg. Chem. 41, 6731–6742 (2002). https://doi.org/10.1021/ic025610+
Liu, X., Xia, W., Jiang, Q., Xu, Y., Yu, P.: Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J. Agric. Food Chem. 62, 297–303 (2014). https://doi.org/10.1021/jf404026f
Xu, B., Kong, X.-L., Zhou, T., Qiu, D.-H., Chen, Y.-L., Liu, M.-S., Yang, R.-H., Hider, R.C.: Synthesis, iron(III)-binding affinity and in vitro evaluation of 3-hydroxypyridin-4-one hexadentate ligands as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 21, 6376–6380 (2011). https://doi.org/10.1016/j.bmcl.2011.08.097
Dash, D., Baral, M., Kanungo, B.K.: Development of a flexible tripodal hydroxypyridinone ligand with cyclohexane framework: complexation, solution thermodynamics spectroscopic and DFT studies. ChemistrySelect. 6, 12165–12181 (2021). https://doi.org/10.1002/slct.202102962
Dash, D., Baral, M., Kanungo, B.K.: Synthesis of a new tetradentate chelator with 1-Hydoroxy-2(1H)-pyridinone (HOPO) as chelating unit: Interaction with Fe (III), solution thermodynamics and DFT studies. J. Mol. Struct. 1222, 128796 (2020). https://doi.org/10.1016/j.molstruc.2020.128796
Toni, M., Massimino, M.L., De Mario, A., Angiulli, E., Spisni, E.: Metal dyshomeostasis and their pathological role in prion and prion-like diseases: the basis for a nutritional approach. Front. Neurosci. (2017). https://doi.org/10.3389/fnins.2017.00003
Fouani, L., Menezes, S.V., Paulson, M., Richardson, D.R., Kovacevic, Z.: Metals and metastasis: exploiting the role of metals in cancer metastasis to develop novel anti-metastatic agents. Pharmacol. Res. 115, 275–287 (2017). https://doi.org/10.1016/j.phrs.2016.12.001
Mocchegiani, E., Giacconi, R., Malavolta, M.: Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol. Med. 14, 419–428 (2008). https://doi.org/10.1016/j.molmed.2008.08.002
Martindale, W., Reynolds, J.E.F.: The extra pharmacopoeia. Royal Pharmaceutical Press, London (1996)
Gemma, S., Kukreja, G., Campiani, G., Butini, S., Bernetti, M., Joshi, B.P., Savini, L., Basilico, N., Taramelli, D., Yardley, V., Bertamino, A., Novellino, E., Persico, M., Catalanotti, B., Fattorusso, C.: Development of piperazine-tethered heterodimers as potent antimalarials against chloroquine-resistant P. falciparum strains. Synthesis and molecular modeling. Bioorg. Med. Chem. Lett. 17, 3535–3539 (2007). https://doi.org/10.1016/j.bmcl.2007.04.077
Santos, M.A., Chand, K., Chaves, S.: Recent progress in multifunctional metal chelators as potential drugs for Alzheimer’s disease. Coord. Chem. Rev. 327–328, 287–303 (2016). https://doi.org/10.1016/j.ccr.2016.04.013
Crisponi, G., Nurchi, V.M.: Metal Ion Toxicity. In: Scott, R.A. (ed.) Encyclopedia of Inorganic and Bioinorganic Chemistry, pp. 1–14. Wiley, Hoboken (2015)
Andersen, O.: Principles and recent developments in chelation treatment of metal intoxication. Chem. Rev. 99, 2683–2710 (1999). https://doi.org/10.1021/cr980453a
Basketter, D.A., Angelini, G., Ingber, A., Kern, P.S., Menné, T.: Nickel, chromium and cobalt in consumer products: revisiting safe levels in the new millennium. Contact Dermatitis 49, 1–7 (2003). https://doi.org/10.1111/j.0105-1873.2003.00149.x
World Health Organization, International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans eds: Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide, and vanadium pentoxide. International Agency for Research on Cancer ; Distributed by WHO Press, Lyon, France : Geneva, Switzerland (2006)
Sunderman, F.W., Decsy, M.I., McNeely, M.D.: Nickel metabolism in health and disease. Ann. NY Acad. Sci. 199, 300–312 (1972). https://doi.org/10.1111/j.1749-6632.1972.tb46465.x
Angelova, M.G., Petkova-Marinova, T.V., Pogorielov, M.V., Loboda, A.N., Nedkova-Kolarova, V.N., Bozhinova, A.N.: Trace element status (Iron, Zinc, Copper, Chromium, Cobalt, and Nickel) in iron-deficiency anaemia of children under 3 years. Anemia 2014, 1–8 (2014). https://doi.org/10.1155/2014/718089
Nielsen, F.H., Shuler, T.R., McLeod, T.G., Zimmerman, T.J.: Nickel influences iron metabolism through physiologic, pharmacologic and toxicologic mechanisms in the rat. J. Nutr. 114, 1280–1288 (1984). https://doi.org/10.1093/jn/114.7.1280
Zhang, Y., Rodionov, D.A., Gelfand, M.S., Gladyshev, V.N.: Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics 10, 78 (2009). https://doi.org/10.1186/1471-2164-10-78
Denkhaus, E., Salnikow, K.: Nickel essentiality, toxicity, and carcinogenicity. Crit Rev OncolHematol. 42, 35–56 (2002). https://doi.org/10.1016/s1040-8428(01)00214-1
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43, 1739–1753 (1996). https://doi.org/10.1016/0039-9140(96)01958-3
Alderighi, L., Gans, P., Ienco, A., Peters, D., Sabatini, A., Vacca, A.: Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 184, 311–318 (1999). https://doi.org/10.1016/S0010-8545(98)00260-4
Irving, H., Williams, R.J.P.: 637. The stability of transition-metal complexes. J. Chem. Soc. (1953). https://doi.org/10.1039/jr9530003192
Nurchi, V.M., Crisponi, G., Lachowicz, J.I., de Jaraquemada-PelaezGuadalupe, M., Bretti, C., Peana, M., Medici, S., Zoroddu, M.A.: Equilibrium studies of new bis-hydroxypyrone derivatives with Fe3+, Al3+, Cu2+ and Zn2+. J InorgBiochem. 189, 103–114 (2018). https://doi.org/10.1016/j.jinorgbio.2018.09.013
Nurchi, V.M., de Guadalupe Jaraquemada-Pelaez, M., Crisponi, G., Lachowicz, J.I., Cappai, R., Gano, L., Santos, M.A., Melchior, A., Tolazzi, M., Peana, M., Medici, S., Zoroddu, M.A.: A new tripodal kojic acid derivative for iron sequestration: Synthesis, protonation, complex formation studies with Fe3+, Al3+, Cu2+ and Zn2+, and in vivo bioassays. J InorgBiochem. 193, 152–165 (2019). https://doi.org/10.1016/j.jinorgbio.2019.01.012
Nurchi, V.M., Crisponi, G., Arca, M., Crespo-Alonso, M., Lachowicz, J.I., Mansoori, D., Toso, L., Pichiri, G., Amelia Santos, M., Marques, S.M., Niclós-Gutiérrez, J., González-Pérez, J.M., Domínguez-Martín, A., Choquesillo-Lazarte, D., Szewczuk, Z., Antonietta Zoroddu, M., Peana, M.: A new bis-3-hydroxy-4-pyrone as a potential therapeutic iron chelating agent. Effect of connecting and side chains on the complex structures and metal ion selectivity. J InorgBiochem. 141, 132–143 (2014). https://doi.org/10.1016/j.jinorgbio.2014.09.002
Kohn, W., Sham, L.J.: Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965). https://doi.org/10.1103/PhysRev.140.A1133
Becke, A.D.: Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J. Chem. Phys. 107, 8554–8560 (1997). https://doi.org/10.1063/1.475007
Bauernschmitt, R., Ahlrichs, R.: Stability analysis for solutions of the closed shell Kohn-Sham equation. J. Chem. Phys. 104, 9047–9052 (1996). https://doi.org/10.1063/1.471637
Pearson, R.G.: Chemical Hardness. Wiley, Hoboken (1997)
Pearson, R.G.: Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. U.S.A. 83, 8440–8441 (1986). https://doi.org/10.1073/pnas.83.22.8440
Pearson, R.G.: Recent advances in the concept of hard and soft acids and bases. J. Chem. Educ. 64, 561 (1987). https://doi.org/10.1021/ed064p561
Koopmans, T.: Über die Zuordnung von Wellenfunktionen und Eigenwertenzu den EinzelnenElektronenEines Atoms. Physica 1, 104–113 (1934). https://doi.org/10.1016/S0031-8914(34)90011-2
Gans, P., Sabatini, A., Vacca, A.: Determination of equilibrium constants from spectrophometric data obtained from solutions of known pH: the program pHab. Anal. Chim. 89, 45–49 (1999)
TeVelde, G., Bickelhaupt, F.M., Baerends, E.J., Fonseca Guerra, C., Van Gisbergen, S.J.A., Snijders, J.G., Ziegler, T.: Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001). https://doi.org/10.1002/jcc.1056
Author information
Authors and Affiliations
Contributions
Shalini Singh: Experimental work, drafting; Minati Baral: Concept, supervision, and manuscript correction; B K Kanungo: validation and correction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Kanungo, B.K. & Baral, M. Coordination competency of a flexible polyfunctional tripodal framework: an insight on solution thermodynamics and DFT studies. J Incl Phenom Macrocycl Chem (2024). https://doi.org/10.1007/s10847-024-01229-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10847-024-01229-1