Abstract
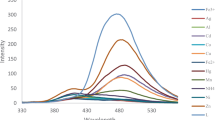
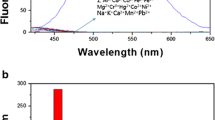
Utilizing a high-dilution condition method, a new aza-oxo macrocycle, referred to as L, was synthesized as a chemosensor. The chemosensing capabilities of L were thoroughly investigated using fluorescence studies. The obtained results demonstrate that L effectively responds to the presence of zinc ions, leading to a significant increase in fluorescence intensity. Comparative studies were conducted to investigate the impact of various metal cations, including Cr(III), Mn(II), Fe(II), Fe(III), Co(II), Ni(II), Cu(I), Cu(II), Zn(II), Cd(II), Gd(III), Na(I), K(I), Ba(II), Mg(II), Al(III), Pb(II), Sn(II), Hg(II), and Ag(I), on the fluorescence of L in an aqueous ethanol environment. During our investigations, a binding constant value of 7.81 × 105 M−1, with a 1:1 stoichiometry for Zn2+–L interactions, was established. Additionally, a low detection limit of 2.51 × 10−8 M and a rapid response time were observed. Furthermore, the chemical inputs of Zn2+ and Cu2+ ions meet the conditions of an INHIBIT molecular logic gate.











Similar content being viewed by others
Data availability
All the data generated or analyzed during this study are included in the published article.
References
Frederickson, C.J., Koh, J.-Y., Bush, A.I.: Nat. Rev. Neurosci. 6, 449 (2005)
Bush, A.I., Pettingell, W.H., Multhaup, G., Paradis, M., Vonsattel, J.-P., Gusella, J.F., Beyreuther, K., Masters, C.L., Tanzi, R.E.: Science 265, 1464 (1994)
Piskacek, M., Zotova, L., Gábor, Z.: J. Cell. Mol. Med. 13, 693–697 (2009)
Kaur, K., Bhardwaj, V.K., Kaur, N., Singh, N.: Inorg. Chem. Commun. 26, 31–36 (2012)
Gupta, V.K., Sethi, B., Sharma, R.A., Agarwal, S., Bharti, A.: J. Mol. Liq. 177, 114–118 (2013)
Gupta, V.K., Karimi-Maleh, H., Sadegh, R.: Int. J. Electrochem. Sci. 10, 303–316 (2015)
Rout, K., Manna, A.K., Sahu, M., Patra, G.K.: Inorg. Chim. Acta 486, 733–741 (2019)
Kim, M.S., GeunJo, T., Yang, M., Han, J., Lim, M.H., Kim, C.: Spectrochim. Acta A Mol. Biomol Spectrosc. 211, 34–43 (2019)
Kim, A., Lee, H., Yun, D., Jung, U., Kim, K.-T., Kim, C.: Spectrochim. Acta. A Mol. Biomol. Spectrosc. 241, 118652 (2020)
So, H., Choa, H., Lee, H., Tran, M.C., Kim, K.-T., Kim, C.: Microchem. J. 155, 104788 (2020)
Maity, D., Mandal, S.K., Guha, B., Roy, P.: Inorg. Chim. Acta 519, 120258 (2021)
Fu, H., Liu, H., Zhao, L., Xiao, B., Fan, T., Jiang, Y.: Tetrahedron 75, 130710 (2019)
Jonaghani, M.Z., Zali-Boeini, H., Moradi, H.: Spectrochim. Acta. A Mol. Biomol. Spectrosc. 207, 16–22 (2019)
Wang, P., Wu, X., Wu, J.: J. Photochem. Photobiol. A Chem. 382, 111929 (2019)
Rani, B.K., John, S.A.: J. Photochem. Photobiol. A Chem. 418, 113372 (2021)
Wang, P., Xue, S.: X, Yang. Microchem. J. 158, 105147 (2020)
Hu, J.-H., Li, J.-B., Qi, J., Sun, Y.: Sens. Actuators B 208, 581–587 (2015)
Khatun, M., Mandal, J., Tamang, V.W., Ghosh Chowdhury, S., Karmakar, P., Saha, A.: J. Photochem. Photobiol. A Chem. 447, 115231 (2024)
Bumagina, N.A., Antina, E.V., Nikonova, A.Y., Berezin, M.B., Ksenofontov, A.A., Vyugin, A.I.: J. Fluoresc. 26, 1967–1974 (2016)
Yun, J.Y., Chae, J.B., Kim, M., Lim, M.H., Kim, C.: Photochem. Photobiol. Sci. 18, 166–176 (2019)
Mandal, J., Jana, N.C., Chowdhury, S.G., Karmakar, P., Saha, A.: Inorg. Chem. Commun. 156, 111217 (2023)
Behura, R., Dash, P.P., Mohanty, P., Behera, S., Mohanty, M., Dinda, R., Behera, S.K., Barick, A.K., Jali, B.R.: J. Mol. Struct. 1264, 133310 (2022)
Azadbakht, R., Hakimi, M., Khanabadi, J.: Spectrochim. Acta A 250, 119236–119241 (2021)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: GAUSSIAN 09, Revision D.01. Gaussian Inc, Wallingford (2009)
Becke, A.D.: J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Phys. Rev. B 37, 785–789 (1988)
Hay, P.J., Wadt, W.R.: J. Chem. Phys. 82, 270–283 (1985)
Wadt, W.R., Hay, P.J.: J. Chem. Phys. 82, 284–298 (1985)
Hay, P.J., Wadt, W.R.: J. Chem. Phys. 82, 299–310 (1985)
Barone, V., Cossi, M.: J. Phys. Chem. A 102, 1995–2001 (1998)
Cossi, M., Barone, V.: J. Chem. Phys. 115, 4708–4717 (2001)
Cossi, M., Rega, N., Scalmani, G., Barone, V.: J. Comput. Chem. 24, 669–681 (2003)
O’Boyle, N.M., Tenderholt, A.L., Langner, K.M.: J. Comput. Chem. 29, 839–845 (2008)
Mondal, P., Sarkar, R., Hens, A., Rajak, K.K.: RSC Adv. 4, 38769–38782 (2014)
Acknowledgements
The authors thankfully acknowledge the Faculty of Chemistry of Bu-Ali Sina University and Ministry of Science Research and Technology of Iran.
Funding
We would like to note that no funding was received for this study.
Author information
Authors and Affiliations
Contributions
Reza Azadbakht and Mostafa Koolivand contributed to the collection of emission spectra, data analysis, and writing the original draft. Hasti Moshiri played a key role in synthesizing the compounds.
Corresponding author
Ethics declarations
Competing interests
All authors, including Reza Azadbakht, Hasti Moshiri, and Mostafa Koolivand, affirm that they have no conflict of interest.
Ethical approval
This article does not involve any studies with human or animal subjects.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azadbakht, R., Moshiri, H. & Koolivand, M. Development of a highly selective and sensitive fluorescent chemosensory for zinc ion detection in aqueous ethanol solution: synthesis of a new aza-oxo macrocycle using high-dilution condition method. J Incl Phenom Macrocycl Chem (2024). https://doi.org/10.1007/s10847-024-01223-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10847-024-01223-7