Abstract
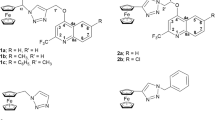
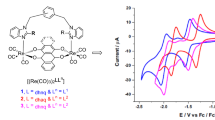
The monitoring of shifting of the redox potential of macrocyclic complexes towards anodic or cathodic regions, which acts as a mediator in many electrocatalytic events, is made possible by inserting electron donating or electron withdrawing group into their frameworks. Herein, using a template strategy, two [14]-membered N4-macrocyclic complexes (denoted as complex A and complex B) with similar molecular cores but different phenyl moieties were prepared and characterized using multiple characterization techniques. The characterization results suggested a saddle-shaped geometry for these complexes, which might be due to the steric repulsions between the benzenoid and amidic moieties on the macrocyclic framework, as also supported by theoretical computations. Further, to investigate the electrochemical behaviors of these complexes, cyclic voltammetry was used and found that the Fe3+/2+ redox potential was systematically shifted in anodic direction with the increment of phenyl moieties on the [14]-membered N4-macrocyclic core. DFT calculations indicated the down-shifting in the most occupied molecular orbital due to the increased phenyl conjugation, which could be correlated with the shifting of Fe3+/2+ redox potential. Biological evaluation of these complexes has also been carried out.







Similar content being viewed by others
References
Abou, D.S., Thiele, N.A., Gutsche, N.T., Villmer, A., Zhang, H., Woods, J.J., Baidoo, K.E., Escorcia, F.E., Wilson, J.J., Thorek, D.L.: Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand. Chem. Sci. 12, 3733–3742 (2021)
Hong, Y., Li, L., Huang, B., Tang, X., Zhai, W., Hu, T., Yuan, K., Chen, Y.: Molecular Control of Carbon-Based Oxygen Reduction Electrocatalysts through Metal Macrocyclic Complexes Functionalization. Adv. Energy Mater. 11, 2100866 (2021)
Kumar, A., Das, D.K., Vashistha, V.K., Ibraheem, S., Yasin, G., Gautam, S., Sharma, V.: A novel CoN4-driven self-assembled molecular engineering for oxygen reduction reaction. Int. J. Hydrog. Energy. 46, 26499–26506 (2021)
Kumar, A., Zhang, Y., Liu, W., Sun, X.: The chemistry, recent advancements and activity descriptors for macrocycles based electrocatalysts in oxygen reduction reaction. Coord. Chem. Rev. 402, 213047 (2020)
Kumar, A., Ibraheem, S., Nguyen, T.A., Gupta, R.K., Maiyalagan, T., Yasin, G.: Molecular-MN4 vs atomically dispersed M? N4? C electrocatalysts for oxygen reduction reaction. Coord. Chem. Rev. 446, 214122 (2021)
Iqbal, R., Akbar, M.B., Ahmad, A., Hussain, A., Altaf, N., Ibraheem, S., Yasin, G., Khan, M.A., Tabish, M., Kumar, A.: Exploring the Synergistic Effect of Novel Ni-Fe in 2D Bimetallic Metal-Organic Frameworks for Enhanced Electrochemical Reduction of CO2. Adv. Mater. Interfaces. 9, 2101505 (2022)
Ni, Y., Lu, Y., Zhang, K., Chen, J.: Aromaticity/antiaromaticity effect on activity of transition metal macrocyclic complexes towards electrocatalytic oxygen reduction. ChemSusChem, 14 1835–1839. (2021)
Mukherjee, A., Ghule, S., Vanka, K.: Computational Insights into the Role of External and Local Electric Fields in Macrocyclic Chemical and Biological Systems. ChemPhysChem. 22, 2484–2492 (2021)
Kumar, A., Vashistha, V.K., Sharma, V.: Substituent effect on catalytic activity of Co phthalocyanines for oxygen reduction reactions, Inorganic Chemistry Communications. Inorg. Chem. Commun. 127, 108518 (2021)
Kumar, A., Yasin, G., Korai, R.M., Slimani, Y., Ali, M.F., Tabish, M., Nazir, M.T., Nguyen, T.A.: Boosting oxygen reduction reaction activity by incorporating the iron phthalocyanine nanoparticles on carbon nanotubes network. Inorg. Chem. Commun. 120, 108160 (2020)
Kumar, A., Zhang, Y., Jia, Y., Liu, W., Sun, X.: Redox chemistry of N4-Fe2+ in iron phthalocyanines for oxygen reduction reaction. Chin. J. Catal. 42, 1404–1412 (2021)
Nath, I., Chakraborty, J., Verpoort, F.: Metal organic frameworks mimicking natural enzymes: a structural and functional analogy. Chem. Soc. Rev. 45, 4127–4170 (2016)
Fouad, R., Shebl, M., Saif, M., Gamal, S.: Novel copper nano-complex based on tetraazamacrocyclic backbone: Template synthesis, structural elucidation, cytotoxic, DNA binding and molecular docking studies. J. Mol. Struct. 1251, 132021 (2022)
Kumar, A., Vashistha, V.K., Tevatia, P., Singh, R.: Electrochemical studies of DNA interaction and antimicrobial activities of MnII, FeIII, CoII and NiII Schiff base tetraazamacrocyclic complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 176, 123–133 (2017)
Vashistha, V.K., Das, D.K., Yadav, A., Saini, D., Kumar, A.: Synthesis, Structure and Catalytic Performance of N4-Macrocycles of Fe III and Co II for Oxidation of Hydroquinone. Anal. Bioanalytical Electrochem. 12, 318–328 (2020)
Hajari, S., Keypour, H., Rezaei, M.T., Farida, S.H.M., Gable, R.W.: New 15-membered macrocyclic Schiff base ligand; synthesis some Cd (II), Mn (II) and Zn (II) complexes, crystal structure, cytotoxicity, antibacterial and antioxidant activity. J. Mol. Struct. 1251, 132049 (2022)
Nikolić, M.A., Dražić, B., Cristóvão, B., Bartyzel, A., Miroslaw, B., Tanasković, S.: New azamacrocyclic binuclear Cu (II) aminocarboxylate complexes: Structural, magnetic, spectral and antiproliferative studies. J. Mol. Struct. 1252, 131969 (2022)
Vashistha, V.K., Kumar, A., Tevatia, P., Das, D.K.: Synthesis, characterization, electrochemical and antimicrobial studies of iron (II) and nickel (II) macrocyclic complexes. Russ. J. Electrochem. 57, 348–356 (2021)
Sharma, N., Kumar, D., Shrivastava, R., Shrivastava, S., Awasthi, K.K.: Synthesis and spectral studies of lanthanide metal tetraaza macrocyclic complexes. Materials Today: Proceedings, 42 1760–1765. (2021)
Kherrouba, A., Bensegueni, R., Guergouri, M., Boulkedid, A.-L., Boutebdja, M., Bencharif, M.: Synthesis, crystal structures, optical properties, DFT and TD-DFT studies of Ni (II) complexes with imine-based ligands. J. Mol. Struct. 1247, 131351 (2022)
Kumar, A., Randhir, R.: Surface-modified Electrodes: Oxidative Electropolymerisation of Macrocyclic Complexes of Fe(III) and Ni(II), Analytical and Bioanalytical Electrochemistry. (2016)
Kumar, A., Tevatia, P., Agarwal, M., Singh, R.: Synthesis, spectral, electrochemical and antibacterial studies of tetraaza macrocyclic complexes of Mn (II) and Co (II). Int. J. Pharm. Chem. 5, 149–157 (2015)
Jirásek, M., Rickhaus, M., Tejerina, L., Anderson, H.L.: Experimental and theoretical evidence for aromatic stabilization energy in large macrocycles. J. Am. Chem. Soc. 143, 2403–2412 (2021)
Jirasek, M., Anderson, H.L., Peeks, M.D.: From Macrocycles to Quantum Rings: Does Aromaticity Have a Size Limit?. Acc. Chem. Res. 54, 3241–3251 (2021)
Rajakkani, P., Alagarraj, A., Thangavelu, S.A.G.: Tetraaza macrocyclic Schiff base metal complexes bearing pendant groups: Synthesis, characterization and bioactivity studies. Inorg. Chem. Commun. 134, 108989 (2021)
Vashistha, V., Kumar, A.: Synthesis of Co(II) and Ni(II) asymmetric tetraazamacrocyclic complexes and their electrochemical and antimicrobial studies. Russ. J. Inorg. Chem. 65, 2028–2032 (2020)
Dey, L., Rabi, S., Palit, D., Hazari, S.K., Begum, Z.A., Rahman, I.M., Roy, T.G.: Syntheses, characterization, and antimicrobial studies of Ni (II), Cu (II), and Co (III) complexes with an N-pendant azamacrocyclic chelator. J. Mol. Struct. 1240, 130579 (2021)
Siddiki, A.N.A., Islam, S., Begum, S., Salam, M.A.: Synthesis, spectral characterization, thermal behavior and biological activities study of ternary metal complexes of alanine and 1, 8-diaminonapthalene with Co(III), Ni(II), Cu(II), Zn(II) and Cd(II). Materials Today: Proceedings, 46 6374–6381. (2021)
Al-Amiery, A.A.: Antimicrobial and antioxidant activities of new metal complexes derived from (E)-3-((5-phenyl-1, 3, 4-oxadiazol-2-ylimino) methyl) naphthalen-2-ol. Med. Chem. Res. 21, 3204–3213 (2012)
Nair, M.S., Arish, D., Johnson, J.: Synthesis, characterization and biological studies on some metal complexes with Schiff base ligand containing pyrazolone moiety. J. Saudi Chem. Soc. 20, S591–S598 (2016)
Avaji, P.G., Kumar, C.V., Patil, S.A., Shivananda, K., Nagaraju, C.: Synthesis, spectral characterization, in-vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. Eur. J. Med. Chem. 44, 3552–3559 (2009)
Beyene, B.B., Mihirteu, A.M., Ayana, M.T., Yibeltal, A.W.: Synthesis, characterization and antibacterial activity of metalloporphyrins: Role of central metal ion. Results in Chemistry. 2, 100073 (2020)
Alhakimi, A.N.: Synthesis, Characterization and Microbicides Activities of N-(hydroxy-4-((4-nitrophenyl) diazenyl) benzylidene)-2-(phenylamino) Acetohydrazide Metal Complexes. Egypt. J. Chem. 63, 1509–1525 (2020)
Chaabane, L., Chahdoura, H., Moslah, W., Snoussi, M., Beyou, E., Lahcini, M., Srairi-Abid, N., Baouab, M.H.V.: Synthesis and characterization of Ni (II), Cu (II), Fe (II) and Fe3O4 nanoparticle complexes with tetraaza macrocyclic Schiff base ligand for antimicrobial activity and cytotoxic activity against cancer and normal cells. Appl. Organomet. Chem. 33, e4860 (2019)
Acknowledgements
The authors are thankful to GLA University, Mathura to provide the infrastructural support for the completion of this study.
Author information
Authors and Affiliations
Contributions
Ravindra collected the data and prepared the manuscript. Prof. Das and Dr. Kumar reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravinder, Das, D.K. & Kumar, A. Phenyl conjugation effect on Fe3+/2+ formal potential of FeN4-macryclic complex. J Incl Phenom Macrocycl Chem 103, 235–243 (2023). https://doi.org/10.1007/s10847-023-01191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-023-01191-4