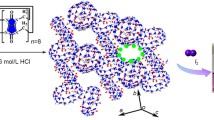
Abstract
The complexation between cucurbit[8]uril, Q[8], and isonicotinic acid has been studied using 1H NMR spectroscopy, UV–Vis absorption spectroscopy, Raman spectroscopy and single crystal X-ray diffraction. The results revealed that the 2:1 inclusion complex (4-PA)2@Q[8]·25H2O is formed, with two guests simultaneously encapsulated in the hydrophobic cavity; the mean planes of the guests are 3.535 Å apart.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isonicotinic acid, also known as 4-pyridinecarboxylic acid (4-PA) is employed in the manufacture of both agrochemicals and pharmaceuticals, and is probably best known for its use in the production of Isoniazid, which is used to treat tuberculosis (TB) [1,2,3,4,5]. Whilst host–guest complexes of 3-pyridinecarboxylic acid (3-PA) with β-cyclodextrin are known [6], those for 4-pyridinecarboxylic acid tend to also involve thiocyanato coordination [7,8,9,10]. Isonicotinate ligands have also been employed as ligands/pillars in 3D arrays [11,12,13], in non-linear optical materials [14, 15], and in luminescent materials [16,17,18]. We are interested in the host–guest properties of cucurbit[n]urils [Q[n]s] [19, 20], and as part of this study, we have been investigated the inclusion ability of the Q[8] system. Given the size of the Q[8] cavity, there is the possibility of the encapsulation of more than one guest. A number of research groups have made use of this possibility, and have accessed intriguing ternary complexes. Examples include the work of Ko et al., [21] that featured the formation of supramolecular assemblies which utilized host-stabilized charge transfer interactions. The same type of interactions has also been exploited to good effect in other work [22,23,24,25,26,27]. To-date, we have investigated the complexation of Q[8] with alkyl substituted 4-pyrrolidinopyridinium salts [28, 29], amino acids [30], and hemicyanine indole [31]. Moreover, in the case of the guests 1′,1″-(alkylene-1,4-diyl)bis(1-butyl)-4,4′-(bipyridine-1,1′-diium)bromide (alkylene = hexylene, octylene), supramolecular polymer formation was achieved via inclusion of alkyl chains [32, 33]. Moreover, we note that inclusion complexes of Isoniazid with tetramethylQ[6] and Q[7] have been reported, for which the guest either resides near the portal or is included in 1:1 fashion respectively [34, 35]. However, in the case of Q[8], poor aqueous solubility means that the presence of hydrochloric acid is often necessary, which in the case of isoniazid results in hydrolysis to isoniacin. Given this, herein, we have extended our studies to the interaction of Q[8] and isonicotinic acid (4-PA), see Fig. 1, and find that Q[8] can accommodate two 4-PA molecules thereby forming the inclusion complex (4-PA)2@Q[8]·25H2O.
Results and discussion
In order to investigate the complexation of Q[8] with 4-PA in solution, 1H NMR spectroscopic titration experiments were performed by adding increasing amounts of Q[8] to the solution of 4-PA in D2O. As can be seen in the insert in Fig. 2, the protons Ha and Hb of the pyridyl ring experience an upfield shift, with that experienced by Hb been the greater (0.06 versus < 0.01 ppm). These observations are consistent with deep inclusion of the pyridyl ring, orientated such that the nitrogen end is nearer the cavity.
To more fully understand the interaction between Q[8] and 4-PA, UV titration experiments were conducted. Displayed in Fig. 3 are the UV spectra obtained for aqueous solutions containing a fixed concentration of 4-PA and variable concentrations of Q[8]. Furthermore, the Job’s plot is shown in Fig. 3c, and is consistent with a stoichiometry of 2:1.
The Raman spectra of the guest, Q[8] and their inclusion complex are presented in Fig. 4a; the detailed Raman assignments for pure Q[8] and complexes are summarized in Tables 1, 2. After complexing with 4-PA, two new peaks appeared near 1000 cm−1, as shown in the Fig. 4b as green circles, which originate from 4-PA. The signature vibration frequency of C–N shifts slightly to higher wavenumbers in the complex as compared to pure Q[8] (Fig. 4c), and this is likely a reflection of the interaction between Q[8] and 4-PA.
Single crystal X-ray diffraction analysis reveals that the compound crystallizes in the tetragonal crystal system, space group I41/a. The asymmetric unit is shown in Fig. 5a, and the inclusion of guests by the complete macrocycle is shown in Fig. 5c; the crystal structure of the complex is composed of the host Q[8] molecule and two guests 4-pyridinecarboxylic acid. Crystallographic data is presented in Table 3. The mean planes of these molecules are 3.535 Å apart. The Q[8] host has encapsulated two isonicotinic acid molecules in an off-set centrosymmetric embrace, bond lengths at the carboxyl group show this is the acid and not carboxylate. Other water was modelled using the Squeeze algorithm as a solvent mask in Olex2. The overall formula includes both ordered and disordered water molecules, and is (4-PA)2@Q[8]·25H2O. As can be seen in Fig. 5b, the encapsulated guest 4-pyridinecarboxylic acid forms multiple hydrogen-bonds with the host Q[8]: the hydrogen-bonding interactions between the carbon atoms of the pyridyl with the portal carbonyl oxygen atoms of Q[8] exhibit distances of C(29)–H⋯O(8) 2.443 Å, C(25)–H⋯O(6) 2.605 Å. The hydrogen of the carboxyl group in isoniacin forms hydrogen bonds with the oxygen atoms of the water molecule with an O(10)–H⋯O(1 W) distance of 1.563 Å. Meanwhile, there is also hydrogen-bonding between the oxygen atom of the water molecule and the portal carbonyl oxygen atoms of Q[8], the distance O(1 W)–H⋯O(5) is 2.092 Å.
a Asymmetric unit with atoms drawn as 30% probability ellipsoids; b Ball-and-stick representation of compound showing guest molecules encapsulated into the Q[8] host, generating a inclusion complex. Most water molecules omitted, only one ordered molecule of water shown here. C = light gray, O = red, N = blue and H = light green; c Molecular structure of (4-PA)2@Q[8]·25H2O; most water molecules omitted for clarity; d Side view of the crystal structures
As shown in Fig. 6, two adjacent complexes form a one-dimensional assembled host–guest supramolecular structure via hydrogen-bonding interactions between the portal carbonyl oxygen atom O5 of Q[8] and the coordinating water molecule O1W. Moreover, the carboxyl oxygen of isoniacin in one complex formed a hydrogen bond with a water molecule and those water molecules formed hydrogen bonds with the carbonyl oxygen of another complex. These hydrogen bonds further strengthen the stabilization of the crystal structure.
The layered two-dimensional structure is formed by one-dimensional supramolecular chains arranged in parallel to each other (Fig. 7). The hydrogen bonding interaction of the adjacent two layers of the supramolecular chains formed via the portal carbonyl oxygen atoms of Q[8] and the coordinating water molecules forms an octagonal hydrogen bonded bridge; this special hydrogen-bonding motif contains four water molecules. Meanwhile, the four water molecules are also involved in hydrogen bonds with the carboxyl oxygen of isoniacin in the four complexes. The resulting hydrogen bonded network results in the extended, stable two-dimensional structure of the complex.
In the crystal stacking diagram of the complex 4-PA@Q[8] along the a-axis direction (Fig. 8a), a supramolecular framework of numerous honeycomb-like channels consisting of one-dimensional supramolecular chain packing is observed; each channel in the framework is surrounded by six host–guest complexes. The framework structure is controlled by the presence of strong and directional hydrogen bonding, forming a two-dimensional network, which act as building blocks and further pack into a three-dimensional framework (Fig. 8b).
Conclusion
In summary, the results herein reveal that Q[8] can simultaneously hold two molecules of 4-pyridinecarboxylic acid in the hydrophobic cavity, both in solution (as shown by 1H NMR and UV–vis spectra) and in the solid-state, as evidenced by a single crystal X-ray diffraction study. In the solid-state, extensive H-bonding results not only in the formation of 1D extended chains, but also 2D and 3D networks; water plays a central role in the formation of these networks.
Experimental section
Materials
General
The guest 4-pyridinecarboxylic acid (4-PA) was obtained from Aladdin (Shanghai, China). Q[8] was prepared and purified according to a literature method [36]. All other reagents were of analytical reagent grade and were used without any further purification. Deionized water was used throughout the experiments.
1H NMR spectroscopy
All the 1H NMR spectra, including those for the titration experiments, were recorded at 298.15 K on a JEOL JNM-ECZ400S 400 MHz NMR spectrometer (JEOL) in D2O. D2O was used as a field-frequency lock and the observed chemical shifts are reported in parts per million (ppm) relative to that for the internal standard (TMS at 0.0 ppm).
UV–Vis absorption spectroscopy
The UV–vis absorption spectra of the host–guest complexes were recorded using an Agilent 8453 spectrophotometer at room temperature. The aqueous solution of 4-PA was prepared with a concentration of 2.4 × 10−3 mol/L. An aqueous solution of Q[8] was prepared with a concentration of 1.0 × 10−3 mol/L for absorption spectra determination. The UV–vis absorption experiments were performed as follows: 500 µL of a 2.4 × 10−3 mol/L stock solution of 4-PA and various amounts of an aqueous 1.0 × 10−3 mol/L Q[8] solution were transferred into a 10 mL volumetric flask, and then the volumetric flask was filled to the final volume with distilled water. Samples of these solutions were combined to give solutions with an NQ[8]/N4-PA = 0, 0.1, 0.2, 0.3, …, and 1.0. The Job’s plot method was used to determine the inclusion ratio of the substance, N4-PA/(NQ[8] + N4-PA) = 0, 0.1, 0.2, 0.3, …, 1.0.
Single-crystal X-ray crystallography
Single-crystal data for compound were collected on the Bruker D8 VENTURE diffractometer with graphite monochromatic Mo-Kα radiation (λ = 0.71073 Å). Empirical absorption corrections were applied by using the multiscan program SADABS. Structural solution and full matrix least-squares refinement based on F2 were performed with the SHELXS-97 and SHELXL-2014 program package, respectively [37, 38]. Anisotropical thermal parameters were applied to all the non-hydrogen atoms. All hydrogen atoms were treated as riding atoms with an isotropic displacement parameter equal to 1.2 times that of the parent atom. Final structure refinement and modelling of the disordered water using a solvent mask were carried out with Olex2 [39].
Preparation of complex
Q[8] (7.54 mg, 0.005 mmol) was added to a solution of 4-pyridinecarboxylic acid (6.15 mg, 0.05 mmol) in HCl solution (5 mL, 6 mol/L). The mixture was heated until complete dissolution. Following slow evaporation of the volatiles from the solution over a period of about 3 weeks, block colorless crystals of complex were obtained.
SERS measurements
Raman spectra were carried out on WITec alpha300R confocal Raman microscopy (WITec GmbH). It operated at a laser (cobalt Laser) wavelength of 532 nm; 600-g/mm grating; scanning time of 30 s, with one accumulation; and laser power of 5 mW. Before the sample measurement, pure Q[8], 4-pyridinecarboxylic acid solid samples, and several better quality complex crystals were prepared, after placing the sample on a slide for compression, the sample can be measured.
Supplementary material
The crystallographic data (excluding structure factors) for the structure in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 2132077.
References
Seydel, J.K., Schaper, K.J., Wempe, E., Cordes, H.P.: Mode of action and quantitative structure-activity correlations of tuberculostatic drugs of the isonicotinic acid hydrazide type. J. Med. Chem. 19, 483–492 (1976). https://doi.org/10.1021/jm00226a007
Bagchi, M.C., Maiti, B.C., Mills, D., Basak, S.C.: Usefulness of graphical invariants in quantitative structure–activity correlations of tuberculostatic drugs of the isonicotinic acid hydrazide type. J. Mol. Model. 10, 102–111 (2004). https://doi.org/10.1007/s00894-003-0173-6
Hu, Y.-Q., Zhang, S., Zhao, F., Gao, C., Feng, L.-S., Lv, Z.-S., Xu, Z., Wu, X.: Isoniazid derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 133, 255–267 (2017). https://doi.org/10.1016/j.ejmech.2017.04.002
Guo, S.-W., Huang, Q.-X., Chen, Y., Wei, J.-W., Zheng, J., Wang, L.-Y., Wang, Y.-T., Wang, R.: Synthesis and bioactivity of guanidinium-functionalized pillar[5]arene as a biofilm disruptor. Angew. Chem. Int. Ed. 60, 618–623 (2021). https://doi.org/10.1002/anie.202013975
Huang, Q.-X., Zhao, H., Shui, M.-J., Guo, D.-S., Wang, R.: Heparin reversal by an oligoethylene glycol functionalized guanidinocalixarene. Chem. Sci. 11, 9623–9629 (2020). https://doi.org/10.1039/D0SC03922E
Saha, S., Roy, A., Roy, K., Roy, M.N.: Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 6, 35764 (2016). https://doi.org/10.1038/srep35764
Sekiya, R., Nishikiori, S.-I.: A preparative strategy for supramolecular inclusion compounds by combination of dimer formation of isonicotinic acid and coordination bonding. Chem. Commun. 24, 2612–2613 (2001). https://doi.org/10.1039/B108458E
Sekiya, R., Nishikiori, S.-I.: Design and structural extension of a supramolecular inclusion-compound host made by the formation of dimers of isonicotinic acid and thiocyanato coordinating bridges. Chem. Eur. J. 8, 4803–4810 (2002). https://doi.org/10.1002/1521-3765(20021018)8:20%3c4803::AID-CHEM4803%3e3.0.CO;2-H
Sekiya, R., Nishikiori, S.-I., Ogura, K.: Crystalline inclusion compounds constructed through self-assembly of isonicotinic acid and thiocyanato coordination bridges. J. Am. Chem. Soc. 126, 16587–16600 (2004). https://doi.org/10.1021/ja0463280
Sekiya, R., Nishikiori, S.-I., Ogura, K.: Coordination framework hosts consisting of 4-pyridyl-substituted carboxylic acid (PCA) dimers and 1D chains of Ni2+ and SCN−: a rational structural extension toward coordination framework hosts with large rectangular cavities. Inorg. Chem. 45, 9233–9244 (2006). https://doi.org/10.1021/ic0605367
Tran, D.T., Fan, X., Brenna, D.P., Zavalij, P.Y., Oliver, S.R.J.: Open metal−organic framework containing cuprate chains. Inorg. Chem. 44, 6192–6196 (2005). https://doi.org/10.1021/ic050024c
Cheng, J.-W., Zhang, J., Zheng, S.-T., Yang, G.-Y.: Linking two distinct layered networks of nanosized {Ln18} and {Cu24} wheels through isonicotinate ligands. Chem. Eur. J. 14, 88–97 (2007). https://doi.org/10.1002/chem.200700600
Zeng, M.-H., Wu, M.-C., Liang, H., Zhou, Y.-L., Chen, X.-M., Ng, S.-W.: 3D homometallic carboxylate ferrimagnet constructed from a manganese(II) succinate carboxylate layer motif pillared by isonicotinate spacers. Inorg. Chem. 46, 7241–7343 (2007). https://doi.org/10.1021/ic700832w
Wang, L., Duan, L., Xiao, D., Wang, E., Hu, C.: Synthesis of novel copper compounds containing isonicotinic acid and/or 2,6-pyridinedicarboxylic acid: third-order nonlinear optical properties. J. Coord. Chem. 57, 1079–1087 (2004). https://doi.org/10.1080/00958970412331281773
Chen, J., Li, S.-K., Wang, Z.-Y., Pan, Y.-T., Wei, J.-W., Lu, S.-Y., Zhang, Q.-W., Wang, L.-H., Wang, R.: Synthesis of an AIEgen functionalized cucurbit[7]uril for subcellular bioimaging and synergistic photodynamic therapy and supramolecular chemotherapy. Chem. Sci. 12, 7727–7734 (2021). https://doi.org/10.1039/D1SC01139A
Chen, W.T., Ying, S.-M., Xu, Y.P., Luo, Q.-Y., Liu, D.-S.: Structure and luminescence of [Tb0.5(C6NO2H5)3(H2O)2]2n·(H3O)4n (ZnCl5)n (ZnCl4)2n. J. Struct. Chem. 52, 631 (2011). https://doi.org/10.1134/S0022476611030279
Bhattacharya, B., Dey, R., Ghoshal, D.: Synthesis, crystal structure and photo luminescent property of a 3D metal-organic hybrid of Cd(II) constructed by two different bridging carboxylate. J. Chem. Sci. 125, 661–666 (2013). https://doi.org/10.1007/s12039-013-0397-7
Sun, C., Wang, Z.-Y., Yue, L.-D., Huang, Q.-X., Cheng, Q., Wang, R.: Supramolecular induction of mitochondrial aggregation and fusion. J. Am. Chem. Soc. 142, 16523–16527 (2020). https://doi.org/10.1021/jacs.0c06783
Lin, R.-L., Liu, J.-X., Chen, K., Redshaw, C.: Supramolecular chemistry of substituted cucurbit[n]urils. Inorg. Chem. Front. 7, 3217–3246 (2020). https://doi.org/10.1039/D0Q100529K
Yang, D., Liu, M., Xiao, X., Tao, Z., Redshaw, C.: Polymeric self-assembled cucurbit[n]urils: synthesis, structures and applications. Coord. Chem. Rev. 434, 213733 (2021). https://doi.org/10.1016/j.ccr.2020.213733
Ko, Y.H., Kim, E., Hwang, I., Kim, K.: Supramolecular assemblies built with host-stabilized charge-transfer interactions. Chem. Commun. 13, 1305–1315 (2007). https://doi.org/10.1039/B615103E
Sindelar, V., Cejas, M.A., Raymo, F.M., Chen, W., Parker, S.E., Kaifer, A.E.: Supramolecular assembly of 2,7-dimethyldiazapyrenium and cucurbit[8]uril: a new fluorescent host for detection of catechol and dopamine. Chem. Eur. J. 11, 7054–7059 (2005). https://doi.org/10.1002/chem.200500917
Pattabiraman, M., Natarajan, A., Kaliappan, R., Mague, J.T., Ramamurthy, V.: Template directed photodimerization of trans-1,2-bis(n-pyridyl)ethylenes and stilbazoles in water. Chem. Commun. 36, 4542–4544 (2005). https://doi.org/10.1039/B508458J
Bush, M.E., Bouley, N.D., Urbach, A.R.: Charge-mediated recognition of N-terminal tryptophan in aqueous solution by a synthetic host. J. Am. Chem. Soc. 127, 14511–14517 (2005). https://doi.org/10.1021/ja0548440
Liu, Y., Yu, Y., Gao, J., Wang, Z., Zhang, X.: Water-soluble supramolecular polymerization driven by multiple host-stabilized charge-transfer interactions. Angew. Chem. Int. Ed. 49, 6576–6579 (2010). https://doi.org/10.1002/anie.201002415
Appel, E.A., Biedermann, F., Rauwald, U., Jones, S.T., Zayed, J.M., Scherman, O.A.: Supramolecular cross-linked networks via host−guest complexation with cucurbit[8]uril. J. Am. Chem. Soc. 132, 14251–14260 (2010). https://doi.org/10.1021/ja106362w
Zhang, K.-D., Tian, J., Hanifi, D., Zhang, Y., Sue, A.C.-H., Zhou, T.-Y., Zhang, L., Zhao, X., Liu, Y., Li, Z.-T.: Toward a single-layer two-dimensional honeycomb supramolecular organic framework in water. J. Am. Chem. Soc. 135, 17913–17918 (2013). https://doi.org/10.1021/ja4086935
Xu, W., Kan, J., Yang, B., Prior, T.J., Bian, B., Xiao, X., Tao, Z., Redshaw, C.: A study of the interaction between cucurbit[8]uril and alkyl-substituted 4-pyrrolidinopyridinium salts. Chem. Asian J. 1, 235–242 (2019). https://doi.org/10.1002/asia.201801498
Xu, W., Deng, X., Xiao, X., Bian, B., Chen, Q., Dalgarno, S.J., Tao, Z., Redshaw, C.: Supramolecular assemblies controlled by cucurbit[n]uril size (n = 6, 7, 8 and 10). New J. Chem. 44, 4311–4318 (2020). https://doi.org/10.1039/D0NJ00087F
Xu, W., Feng, H., Zhao, W., Shan, P., Huang, C., Redshaw, C., Tao, Z., Xiao, X.: Amino acid recognition by a fluorescent chemosensor based on cucurbit[8]uril and acridine hydrochloride. Anal. Chim. Acta 1135, 142–149 (2020). https://doi.org/10.1016/j.aca.2020.09.028
Xu, X., Kan, J., Redshaw, C., Bian, B., Fan, Y., Tao, Z., Xiao, X.: A hemicyanine and cucurbit[n]uril inclusion complex: competitive guest binding of cucurbit[7]uril and cucurbit[8]uril. Supramol. Chem. 31, 457–465 (2019). https://doi.org/10.1080/10610278.2019.1624748
Xiao, X., Liu, J.-X., Zhu, Q.-J., Xue, S.-F., Tao, Z.: Metal cation controlled supramolecular assembly of 1-butyl-4,4′-bipyridinium and cucurbit[8]uril. Eur. J. Inorg. Chem. 19, 2956–2961 (2010). https://doi.org/10.1002/ejic.201000020
Xiao, X., Sun, N., Qi, D., Jiang, J.: Unprecedented cucurbituril-based ternary host–guest supramolecular polymers mediated through included alkyl chains. Polym. Chem. 5, 5211–5217 (2014). https://doi.org/10.1039/C4PY00512K
Gao, Z.-Z., Bai, D., Xiao, Z.-Y., Zhu, Q.-J., Xue, S.-F., Tao, Z., Xiao, X.: Host–guest interactions in tetramethyl-cucurbit[6]uril with anti-tuberculosis drug isoniazid. Inorg. Chem. Commun. 71, 68–72 (2016). https://doi.org/10.1016/j.inoche.2016.07.005
Wheate, N.J., Vora, V., Anthony, N.G., McInnes, F.J.: Host–guest complexes of the antituberculosis drugs pyrazinamide and isoniazid with cucurbit[7]uril. J. Incl. Phenom. Macrocycl. Chem. 68, 359–367 (2010). https://doi.org/10.1007/s10847-010-9795-3
Day, A., Arnold, A.P., Blanch, R.J., Snushall, B.: Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 66, 8094–8100 (2001). https://doi.org/10.1021/jo015897c
Sheldrick, G.M.: A short history of SHELX. Acta Crystallogr. Sect. A 64, 112–122 (2008). https://doi.org/10.1107/S0108767307043930
Sheldrick, G.M.: Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 71, 3–8 (2015). https://doi.org/10.1107/S2053229614024218
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009). https://doi.org/10.1107/S0021889808042726
Acknowledgements
This work was supported by the Innovation Program for High-level Talents of Guizhou Province (No. 2016-5657), “Chun-Hui” Fund of Chinese Ministry of Education (Z2017005, Z2017001). CR thanks the EPSRC for the award of a travel Grant (EP/R023816/1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Zc., Lu, Y., Shan, Ph. et al. A study of the inclusion complex formed between cucurbit[8]uril and isonicotinic acid. J Incl Phenom Macrocycl Chem 102, 619–628 (2022). https://doi.org/10.1007/s10847-022-01141-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-022-01141-6