Abstract
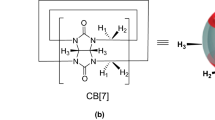
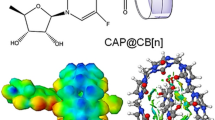
Benznidazole (BNZ) is one of the most recommended drugs for the acute phase of Chagas disease. However, its use has some limitations like low aqueous solubility, low biodisponibility, and considerable toxicity. To overcome these shortcomings, the use of nanocarrier agents is an interesting strategy that has been largely used in drug delivery. Therefore, herein molecular dynamics (MD) simulations and potential of mean force (PMF) technique were used to study the encapsulation of the BNZ into β-cyclodextrin (β-CD) and curcubit[7]uril (CB[7]) cavities in aqueous solution. Along the 50 ns of MD trajectory, the BNZ kept complexed with CB[7] and β-CD without significantly altering their structures and their second solvation shell. In the encapsulation process, the BNZ excluded 6 and 7 water molecules from the interior of CB[7] and β-CD, respectively. Both hosts were able to encapsulate the hydrophobic and hydrophilic groups of the BNZ guest. However, the PMF calculations showed that the BNZ@CB[7] complex is almost three times more stable than the BNZ@β-CD complex, with binding energies respectively equal to − 60.8 and − 21.8 kJ mol−1. Therefore, we highlight the CB[7] as a new macrocyclic host for drug delivery of BNZ that may be more efficient than the β-CD.
Graphical abstract







Similar content being viewed by others
References
Chatelain, E.: Chagas disease drug discovery: toward a new era. J. Biomol. Screen. 20, 22–35 (2015)
Docampo, R.: Recent developments in the chemotherapy of Chagas disease. Curr. Pharm. Des. 7, 1157–1164 (2001)
Maya, J.D., Cassels, B.K., Iturriaga-Vasquez, P., Ferreira, J., Faúndez, M., Galanti, N., Ferreira, A., Morello, A.: Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp. Biochem. Physiol. Part A 146(146), 601–620 (2007)
Pinheiro, E., Brum-Soares, L., Reis, R., Cubides, J.-C.: Chagas disease: review of needs, neglect, and obstacles to treatment access in Latin America. Rev. Soc. Bras. Med. Trop. 50, 296–300 (2017)
Caldas, I.S., Talvani, A., Caldas, S., Carneiro, C.M., da de LanaMatta Guedes, M.P.M., Bahia, M.T.: Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol. Res. 103, 413–421 (2008)
Mazzeti, A.L., Oliveira, L.T., Gonçalves, K.R., Schaun, G.C., Mosqueteira, V.C.F., Bahia, M.T.: Benznidazole self-emulsifying delivery system: A novel alternative dosage form for Chagas disease treatment. Eur. J. Pharm. Sci. 145, 105234 (2020)
Silva, A.M.S., Caland, L.B., Doro, P.N.M., Oliveira, A.L.C.S.L., Aráujo-Júnior, R.F., Fernandes-Pedrosa, M.F., Egito, E.S.T., Silva-Junior, A.A.: Hydrophilic and hydrophobic polymeric benznidazole-loaded nanoparticles: physicochemical properties and in vitro antitumor efficacy. J. Drug Deliv. Sci. Technol. 51, 700–707 (2019)
Seremeta, K.P., Arrúa, E.V., Okulik, N.B., Salomon, C.J.: Development and characterization of benznidazole nano- and microparticles: a new tool for pediatric treatment of Chagas disease? Colloids Surf. B Biointerfaces 177, 169–177 (2019)
Nhavene, E.P.F., da SilvaJunior, W.M.R.R.T., Gastelois, P.L., Venâncio, T., Nascimento, R., Batista, R.J.C., Machado, C.R., Macedo, W.A.A., de Sousa, E.M.B.: Chitosan grafted into mesoporous silica nanoparticles as benznidazol carrier for Chagas diseases treatment. Microporous Mesoporous Mater. 272, 265–275 (2018)
Morilla, M.J., Benavidez, P., Lopez, M.O., Bakas, L., Romero, E.L.: Development and in vitro characterisation of a benznidazole liposomal formulation. Int. J. Pharm. 249, 89–99 (2002)
Vinuesa, T., Herráez, R., Oliver, L., Elizondo, E., Acarregui, A., Esquisabel, A., Pedraz, J.L., Ventosa, N., Veciana, J., Viñas, M.: Benznidazole nanoformulates: a chance to improve therapeutics for Chagas disease. Am. J. Trop. Med. Hyg. 97, 1469–1476 (2017)
Oliveira, E.C.V., Carneiro, Z.A., de Albuquerque, S., Marchetti, J.M.: Development and evaluation of a nanoemulsion containing ursolic acid: a promising trypanocidal agent. AAPS PharmSciTech. 18, 2551–2560 (2017)
Vermelho, A.B., Cardoso, V.D., Ricci, E., Dos Santos, E.P., Supuran, C.T.: Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of Trypanosoma cruzi. J. Enzyme Inhib. Med. Chem. 33, 139–146 (2017)
Streck, L., Sarmento, V.H., de Menezes, R.P., Fernandes-Pedrosa, M.F., Martins, A.M., da Silva-Júnior, A.A.: Tailoring microstructural, drug release properties, and antichagasic efficacy of biocompatible oil-in-water benznidazol-loaded nanoemulsions. Int. J. Pharm. 555, 36–48 (2019)
Arrúa, E.C., Seremeta, K.P., Bedogni, G.R., Okulik, N.B., Salomon, C.J.: Nanocarriers for effective delivery of benznidazole and nifurtimox in the treatment of Chagas disease: areview. Acta Trop. 198, 105080 (2019)
Quezada, C.Q., Azevedo, C.S., Charneau, S., Santana, J.M., Chorilli, M., Carneiro, M.B., Bastos, I.M.D.: Advances in nanocarriers as drug delivery systems in Chagas disease. Int. J. Nanomed. 14, 6407–6424 (2019)
Vikas, Y., Sandeep, K., Braham, D., Manjusha, C., Budhwar, V.: Cyclodextrin complexes: an approach to improve the physicochemical properties of drugs and applications of cyclodextrin complexes. Asian J. Pharm. 12, S3984 (2018)
Carneiro, S.B., Duarte, F.I.C., Heimfarth, L., Quintans, J.S.S., Quintans-Júnior, L.J., Júnior, V.L.V., Lima, A.A.N.: Cyclodextrin–drug inclusion complexes: in vivo and in vitro approaches. Int. J. Mol. Sci. 20, 64 (2019)
Español, E.S., Villamil, M.M.: Calixarenes: generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules 9, 90 (2019)
Assaf, K.I., Nau, W.M.: Cucurbiturils: from synthesis to high-affinity, binding and catalysis. Chem. Soc. Rev. 44, 394 (2015)
Yang, K., Pei, Y., Wen, J., Pei, Z.: Recent advances in pillar[n]arenes: synthesis and application based on host-guest interactions. Chem. Commun. 52, 9316–9326 (2016)
Sobrinho, J.L.S., Soares, M.F.L.R.: Improving the solubility of the antichagasic drug benznidazole through formation of inclusion complexes with cyclodextrins. Quim. Nova 34, 1534–1538 (2011)
Lyra, M.A.M., Soares-Sobrinho, J.L., Figueiredo, R.C.B.Q., Sandes, J.M., Lima, A.A.N., Tenório, R.P., Fontes, D.A.F., Santos, F.L.A., Rolim, L.A., Rolim-Neto, P.J.: Study of benznidazole–cyclodextrin inclusion complexes, cytotoxicity and trypanocidal activity. J. Incl. Phenom. Macrocycl. Chem. 73, 397–404 (2012)
Melo, P.N., Barbosa, E.G., Caland, L.B., Carpegianni, H., Garnero, C., Longhi, M., Fernades-Pedrosa, M.F., Silva-Júnior, A.A.: Host–guest interactions between benznidazole and beta-cyclodextrin in multicomponent complex systems involving hydrophilic polymers and triethanolamine in aqueous solution. J. Mol. Liq. 186, 147–156 (2013)
Bruschi, M.L.: Strategies to modify the drug release from pharmaceutical systems: 6—drug delivery systems. Elsevier, New York (2015)
Kim, K., Murray, J., Selvapalam, N., Ko, Y. H., Hwang, I.: Cucurbiturils: syntheses, structures and properties. In: Cucurbiturils: Chemistry, Supramolecular Chemistry and Applications. World Scientific, Press, Singapore, 2018
Das, D., Assaf, K.I., Nau, W.M.: Applications of cucurbiturils in medicinal chemistry and chemical biology. Front. Chem. 7, 619 (2019)
Gadde, S., Batchelor, E.K., Kaifer, A.E.: Electrochemistry of redox active centres encapsulated by non-covalent methods. Aust. J. Chem. 63, 184–194 (2010)
Ahmadian, N., Mehrnejad, F., Amininasab, M.: Molecular insight into the interaction between camptothecin and acyclic cucurbit[4]urils as efficient nanocontainers in comparison with cucurbit[7]uril: molecular docking and molecular dynamics simulation. J. Chem. Inf. Model. 60, 1791–1803 (2020)
Albdallah, S.K., Assaf, K.I., Bodoor, K., Al-Sakhen, N.A., Malhis, L.D., Alhmaideen, A.I., El-Barghouthi, M.I.: Cucurbit[7]uril inclusion complexes with benzimidazole derivatives: a computational study. J. Solut. Chem. 47, 1768–1778 (2018)
Villarroel-Lecourt, G., Carrasco-Carvajal, J., Andrade-Villalobos, F., So-lís-Egaña, F., Martín, I.M.-S., Robinson-Duggon, J., Fuentealba, D.: Encapsulation of chemotherapeutic drug melphalan in cucurbit[7]uril: effects on its alkylating activity, hydrolysis and cytotoxicity. ACS Omega 3, 8337–8343 (2018)
Suliman, F.O., Varghese, B.: Inclusion complexes of pantoprazole with β-cyclodextrin and cucurbit[7]uril: experimental and molecular modeling study. J. Incl. Phenom. Macrocycl. Chem. 91, 179–188 (2018)
Jorgensen, W.L., Maxwell, D.S., Tirado-Rives, J.: Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996)
Hanwell, M.D., Curtis, D.E., Lonie, D.C., Vandermeersch, T., Zurek, E., Hutchison, G.R.: Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminf. 4, 17 (2012)
Kim, J., Jung, I.-S., Kim, S.-Y., Lee, E., Kang, J.-K., Sakamoto, S., Yamaguchi, K., Kim, K.: New cucurbituril homologues: Syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 122, 540–541 (2000)
Seidel, R.W., Koleva, B.B.: β-cyclodextrin 1041-hydrate. Acta Crystallogr. E65, o3162–o3163 (2009)
Neese, F.: ORCA—An Ab Initio, DFT and Semiempirical SCF-MO Package. Max Planck Institut für Strahlenchemie, Mülheim (2010)
Lu, T., Chen, F.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012)
Ribeiro, A.A.S.T., Horta, B.A.C., de Alencastro, R.B.: MKTOP: a program for automatic construction of molecular topologies. J. Braz. Chem. Soc. 19, 1433–1435 (2008)
DeLano, W.L.: The PyMOL Molecular Graphics System. DeLano Scientific, San Carlos, CA (2009)
Berendsen, H.J.C., Postama, J.P.M., Van Gunsteren, W.F.: Intermolecular forces. In: B. Pullman, ed., Reidel, Dordrecht, 1981
Berendsen, H.J.C., Postma, J.P.M., van Gunsteren, W.F., DiNola, A., Haak, J.R.: Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984)
Bussi, G., Donadio, D., Parrinello, M.: Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007)
Berendsen, H.J.C.: Simulating the physical World: Hierarchial Modeling from Quantum Mechanics to Fluid Dynamics. Cambridge University Press, New York (2007)
Deserno, M., Holm, C.: How to mesh up Ewald sums: I: A theoretical and numerical comparison of various particle mesh routines. J. Chem. Phys. 109, 7678–7693 (1998)
Morse, P.M., Feshbach, H.: Asymptotic Series, Method of Steepest Descent: Methods in Theoretical Physics Part I, pp. 434–443. McGraw-Hill, New York (1953)
Hess, B.: P-LINCS: a parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 4, 116–122 (2008)
Berendsen, H.J.C., van der Spoel, D., van Drunen, R.: GROMACS: a message-passing parallel molecular dynamics implementation. Comput. Phys. Comm. 91, 43–56 (1995)
Hess, B., Kutzner, C., van der Spoel, D., Lindahl, E.: GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008)
Kumar, S., Rosenberg, J.M., Sweden, D., Kolman, R.H.: The weighted histogram analysis method for free energy calcularions on biomolecules: I: The method. J. Comput. Chem. 13, 1011–1021 (1992)
Hub, J.S., de Groot, B.L., van der Spoel, D.: g_wham—a free weighted histogram analysis implementation including robust error and autocorrelation estimates. J. Chem. Theory Comput. 6, 3713–3720 (2010)
Priotti, J., Ferreira, M.J.G., Lamas, M.C., Leonardi, D., Salomon, C.J., Nunes, T.G.: First solid-state NMR spectroscopy evaluation of complexes of benznidazole with cyclodextrin derivatives. Carbohyd. Polym. 131, 90–97 (2015)
Espinosa, Y.R., Galvis-ovallos, F., Rozo, A.M.: Purification of the antichagasic benznidazole from the commercial preparation Rochegan: characterization of inclusion complexes with β-cyclodextrin. J. Cienc. Ing. 10, 32–38 (2018)
Soares-Sobrinho, J.L., de La Roca Soares, M.F., Rolim-Neto, P.J., Torres-Labandeira, J.J.: Physicochemical study of solid-state benznidazole–cyclodextrin complexes. J. Therm. Anal. Cal. 106, 319–325 (2011)
De Lima, A.A.N., Sobrinho, J.L.S., De Lyra, M.A.M., Dos Santos, F.L.A., Figueirêdo, C.B.M., Neto, P.J.R.: Evaluation of in vitro dissolution of benznidazole and binary mixtures: solid dispersions with hydroxypropylmethylcellulose and β-cyclodextrin inclusion complexes. Int. J. Pharm. Pharm. Sci. 7, 371–375 (2015)
Malaspina, T., Fileti, E., Chaban, V.V.: Peculiar aqueous solubility trend in cucurbiturils unraveled by atomistic simulations. J. Phys. Chem. B 120, 7511–7516 (2016)
Biedermann, F., Uzunova, V.D., Scherman, O.A., Nau, W.M., De Simone, A.: Release of high-energy water as an essential driving force for the high-affinity binding of cucurbit[n]urils. J. Am. Chem. Soc. 134, 15318 (2012)
Venkataramanan, N.S., Suvitha, A., Sahara, R.: Structure, stability, and nature of bonding between high energy water clusters confined inside cucurbituril: A computational study. Comput. Theor. Chem. 1148, 44–45 (2019)
Grishaeva, T.N., Masliy, A.N., Kuznetsov, A.M.: Water structuring inside the cavities of cucurbit[n]urils (n = 5–8): a quantum-chemical forecast. J. Incl. Phenom. Macrocycl. Chem. 89, 299–313 (2017)
Pereva, S., Nikolova, V., Angelova, S., Spassov, T., Dudev, T.: Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding. Beilstein J. Org. Chem. 15, 1592–1600 (2019)
Mixcoha, E., Campos-Teran, J., Piñeiro, A.: Surface adsorption and bulk aggregation of cyclodextrins by computational molecular dynamics simulations as a function of temperature: α-CD vs β-CD. J. Phys. Chem. B 118, 6999–7011 (2014)
Jana, M., Bandyopadhyay, S.: Hydration properties of α-, β-, and γ-cyclodextrins from molecular dynamics simulations. J. Phys. Chem. B 115, 6347–6357 (2011)
You, W., Tang, Z., Chang, C.A.: Potential mean force from umbrella sampling simulations: what can we learn and what is missed? J. Chem. Theory Comput. 15, 2433–2443 (2019)
Chadha, R., Jain, D.V.S., Aggarwal, A., Singh, S., Thakur, T.: Binding constants of inclusion complexes of nitroimidazoles with β-cyclodextrins in the absence and presence of PVP. Thermochim. Acta 459, 111–115 (2007)
Soares-Sobrinho, J.L., Santos, F.L.A., Lyra, M.A.M., Alves, L.D.S., Rolim, L.A., Lima, A.A.N., Nunes, L.C.C., Soares, M.F.R., Rolim-Neto, P.J., Torres-Labandeira, J.J.: Benznidazole drug delivery by binary and multicomponent inclusion complexes using cyclodextrins and polymers. Carbohyd. Polym. 89, 323–330 (2012)
Acknowledgements
This research was carried out with the support of the FAPESP (São Paulo Research Foundation) under process number of 2018/19844-8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira, O.V., Viegas, R.G. Cucurbit[7]uril as a possible nanocarrier for the antichagasic benznidazole: a computational approach. J Incl Phenom Macrocycl Chem 98, 93–103 (2020). https://doi.org/10.1007/s10847-020-01014-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-01014-w