Abstract
This review covers construction and properties of porous molecular crystals (PMCs) constructed through hydrogen-bonding of C3-symmetric, rigid, π-conjugated molecular building blocks possessing carboxyaryl groups, which was reported in the last 5 years by the author’s group. PMCs with well-defined, self-standing pores have been attracted attention due to various functionalities provided by selective and reversible inclusion of certain chemical species into the pores. However, it has been recognized for long time that construction of PMCs with permanent porosity is not easy due to weakness of noncovalent intermolecular interactions. Systematic construction of PMCs have been limited so far. To overcome this problem, the author has proposed a unique molecular design concept based on C3-symmetric π-conjugated molecules (C3PIs) possessing o-bis(4-carboxyphenyl)benzene moieties in their periphery and demonstrated that C3PIs systematically yielded hydrogen-bonded organic frameworks (HOFs) composed of H-bonded 2D hexagonal networks (H-HexNets) or interpenetrated 3D pcu-networks, which exhibit permanent porosity, significant thermal stability, polar solvent durability, robustness/flexibility, and/or multifunctionality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: porous molecular crystals
During the past 2 decades, porous materials containing organic components have come to the forefront of science and technologies. Applying designable organic molecules into construction of porous frameworks enables tailored functionalization of the frameworks for purposes such as selective gas storage/separation, catalysts, chemical sensing, ion/proton conducting, optoelectronics, and so on [1]. Metal-organic frameworks (MOFs) [2,3,4,5,6] and covalent-organic frameworks (COFs) [7,8,9,10,11,12] are widely investigated for such applications because of their shape-persistent rigid frameworks connected through dative or covalent bonds and high designability from structural and electronic aspects. There is also renascence in the field of porous molecular crystals (PMCs), which are constructed from molecules through reversible intermolecular interactions [13,14,15,16,17,18].
PMCs are crystalline materials with permanent porosity constructed from discrete organic molecules through noncovalent intermolecular interactions. The origin of the PMC chemistry may be back to Barrer’s and Shanson’s work [19] on Dianin’s compound (4-p-hydroxyphenyl-2,3,4-trimethylchroman), which strongly indicates potential of organic molecules for porous materials such as zeolites. A pioneering works of the recent active phase of PMC chemistry are, for example, those on tris-o-phenylenedioxycyclotriphosphazene (TPP) [20, 21], tetrapyridone derivative [22], cyclic urea [23], and so on [24,25,26,27]. Other excellent PMCs have been also constructed from recently emerged novel cyclic compounds, such as pillararene [28] and cycloparaphenylene [29]. Although PMCs are closely related to classic organic inclusion crystals [30,31,32], an important feature of PMCs is that they have self-standing pores (i.e. permanent porosity) that can accommodate various and/or specific guest molecules reversibly.
Specifically PMCs that are formed via hydrogen-bonding are often called as hydrogen-bonded organic frameworks (HOFs) [33], supramolecular organic frameworks (SOFs) [34, 35], or porous organic salts (POSs) [36], depending on what the authors want to focus on. Although several names and acronyms to describe PMCs constructed through H-bonding can be found in the literature, the name HOF is applied in the later part of this review article. Typical features of HOFs, compared with other porous materials such as MOFs and COFs, are as follows [37,38,39,40,41]:
-
(1)
High crystallinity with large domain: HOFs are frequently obtained as a single crystal via a simple solution process thanks to the reversible H-bond formation. This enables us to determine the precise crystal structure by single-crystal X-ray diffraction (SXRD).
-
(2)
Environmental friendliness: No heavy or transition metal species are necessary for framework construction, which can also provide lightweight materials. Moreover, the materials have ability to restore crystallinity by reannealing and are easily re-generated via a solution process.
-
(3)
Flexibility: Rearrangement of H-bonds can provide structural changes to generate functionality.
Such intrinsic properties of HOFs, however, simultaneously cause the following problems. Namely, HOFs tend to collapse during activation (removing of solvent molecules from voids). HOFs are not easy to design as chemists want to: Even when molecules are carefully designed, the desired porous HOFs do not always produce, but other structures such as nonporous crystals do. Inconveniently, both these two contradictory features come from the same origin. Namely, HOFs are constructed via reversible weak H-bonds. Therefore, we have needed to solve this dilemmas. One of the key ideas to construct stable porous HOFs is combining other secondary intermolecular interactions working between large surfaces, such as π/π interactions, with H-bonds. Another is to apply strong H-bonds such as charge assisted H-bonds [42]. More recently, Cooper and Day demonstrated HOF production based on “energy-structure-function maps” built by combining computational crystal structure prediction and property prediction [43, 44], which has a potential of game-changing technology for development of crystalline functional materials.
Recent progress in the field has produced excellent HOFs, possessing permanent porosity with large surface area, significant thermal and chemical durability, and functionality [45, 27, 46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. For example, In 2012, Mastalerz and Oppel reported that a molecule possessing triptycene skeleton and cyclic urea formed highly porous HOF TTBI with density of 0.755 g cm− 3 and significantly large Brunauer–Emmett–Teller surface area [SA(BET)] [69] of 2796 m2g− 1 [48]. Furthermore, Cooper, Day, and coworkers recently reported a honeycomb HOF with Mastalerz’s molecule [43]. The HOF has an extremely low density of 0.412 g cm− 3, good thermal stability up to 227 °C, and surface area with SA(BET) of 3425 m2g− 1. Chen and coworkers constructed many kinds of HOFs by using diaminotriazine (DTA) as an H-bonding unit and demonstrated selective gas sorption properties [33, 53, 54, 56, 59, 63, 67]. Miljanić and coworkers reported HOFs with fluorinated pore surfaces based on C3-symmetric tripyrazole derivatives [51, 66].
In this review, the author introduces HOFs and related networked frameworks constructed through dimerization of carboxy groups reported by the author’s group in the last 5 years.
Networked structures connected by carboxylic acid dimers
A H-bonded dimer of carboxy groups [70] is one of the simplest and the most popular supramolecular synthon [71] to make molecular assemblies. Meanwhile, the dimer has still been attractive to construct exotic HOFs, because of facile synthesis of derivatives with carboxy groups and its high directional H-bond formation. As shown in Fig. 1, geometrically-well defined building blocks possessing carboxy groups can reasonably form the corresponding topological and geometrical networked structures. In 1969, Marsh and Duchamp demonstrated that trimesic acid 1 yielded a waved H-bonded honeycomb network [72]. The networks were interpenetrated to yield a nonporous crystal, and it was in 1987 that layered honeycomb structures with 1D inclusion channels was constructed by Herbestein and coworkers by templated-crystallization [73]. In 2000, Kobayashi and coworkers demonstrated that hexakis(4-carboxyphenyl)benzene 2 also gave hexagonally-networked 2D sheets, which accumulate layer-by-layer without interpenetration [74]. These two representative classic works strongly imply promising potential of carboxy groups to construct porous frameworks.
Carboxylic acid-based HOFs with permanent porosity have started to be reported intensively since around 2015 (Fig. 2). Rowsell, Zentner et al. reported that 1,3,5-tris(4-carboxyphenyl)benzene (3) can form 2D honeycomb network, which interpenetrate to give the porous HOF (tcpb) [75, 76]. Chen et al. demonstrated that the 3D networked porous framework (HOF-11) formed by tris(4-carboxyphenyl)amine (4) showed SA(BET) of 687 m2g− 1 [59, 57]. Dynamic gas sorption behavior of TCF-1 and -2 composed of tetrahedral building blocks 5a and 5b were reported by Comotti, et al. [77]. The author and coworkers demonstrated that a series of X-shaped molecules 6–8 systematically formed a series of quasi-isostructural rhombic frameworks [78]. Wu et al. reported that biphenyl derivative 9 gave the stable, interpenetrated, 3D-networked framework (HOF-TCBP) with SA(BET) of 2066 m2g− 1 [79]. Liu et al. constructed the pylene-based HOF (PFC-1) with SA(BET) of 2122 m2g− 1 from 10 and demonstrated proof-of-concept for chemo and photodynamic therapy [80]. HOF JLU-SOF-1-R composed of the homochiral 11 had an SA(BET) value of 460 m2g− 1 and selective sorption toward CO2 [81]. Takeda et al. demonstrated that twisted cyclic tetrathienylene 12 yielded a jumping HOF [82]. Stoddart et al. reported that triptycene derivative 13a and 13b provided interpenetration polymorphs of H-bonded networks [83, 84]. It is remarkable that building block molecules recently reported possess carboxyphenyl groups, instead of carboxy groups. This is probably because chemical modification of rigid π-conjugated systems with carboxyphenyl or carboxyaryl groups became much easier than before due to development of facile metal-catalyzed cross-coupling reactions such as Suzuki-Miyaura reaction [85]. Moreover, solubility of the molecule in common organic solvents such as alcohol and N,N-dimethylformamide (DMF) can be improved when a carboxyphenyl group is applied instead of a carboxy group [86, 87]. The phenylene spacer is also effective to generate void spaces in crystals [88,89,90].
The author’s concepts for systematic construction of HOFs
In order to construct isostructural HOFs in a systematic way, the author has proposed the following working hypothesis in 2015 (Fig. 3a). That is that C3-symmetric π-conjugated planar building blocks (C3PIs) possessing three o-bis(4-carboxyphenyl)benzene moieties in periphery can form an isostructural H-bonded hexagonal network (H-HexNet) sheet via H-bonding dimerization of the carboxy groups, and that the H-HexNets are stacked to give porous layered assembly of HexNets (LA-H-HexNets) [91]. In this hypothesis, the key motif is a H-bonded triangular porous motif formed by the peripheral three o-bis(4-carboxyphenyl)benzene moieties. Although the motif has already been observed in Kobayashi’s framework [74], we noticed its importance on constructing HOFs and named the motif as phenylene triangle (PhT) motif [91]. The ring size of PhT motif is appropriate to retain the network structure within crystals. Indeed, if 4-carboxyphenyl groups are replaced by 4-carboxybiphenyl group in order to expand the triangle motif, crystallinity of the resultant LA-H-HexNets drastically decreases.
Furthermore, during investigation on HOFs with planer C3PI molecules, we came to across unexpected results that a hexaazatriphenylene (HAT) derivative with 4-carboxyphenyl groups in the periphery formed no layered HOF (LA-H-HexNet) but a 3D networked HOF (Fig. 3b) [92]. In the crystalline state, packing force makes a HAT core twisted, resulting that the peripheral six carboxyphenyl groups are alternately directed up and down, and consequently, 3D H-bonded network with primitive cubic (pcu) topology is formed. The whole crystal structure is significantly rigid due to both interpenetration of the 3D network and shape-fitted tight docking among the propeller-shaped π-conjugated cores. Particularly the concept “shape-fitted docking” is a key for construction of highly-stable isostructural HOFs. Figure 3c shows various C3PIs surveyed to date in the author’s group. In the following sections, the author describes structures and properties of HOFs that are constructed from these C3PIs based on the above mentioned concepts in detail.
Concepts of the author’s work. a Construction of layered assembly of H-boned hexagonal networks (LA-H-HexNet) with planar C3PIs via formation of cyclic H-bonded motifs, so-called phenylene triangle (PhT) motifs. b Construction of 3D networked rigid HOFs with non-planar C3PI conformers via formation of helical H-bonded motifs, interpenetration of the networks, and shape-fitted-docking of the core. c C3PIs surveyed in the author’s group
Structural features of the phenylene triangle (PhT) motif
As described above, the PhT motif is a key for HOFs with H-HexNet structures. In this section, therefore, its structural features will be described (Fig. 4). The PhT, which possesses a triangular void with ca. 12.5 Å on a side and ca. 9.7 Å of diameter, includes conformational frustration originated from steric hindrance of the o-terphenyl moiety. The two peripheral phenylene groups in the ortho-position prefer to incline in the same direction (P or M) to avoid steric repulsion between them (Fig. 4a). As a result, when three o-terphenyl derivatives form the PhT motif, the motif involves at least one conformationally-frustrated H-bonded dimer of the carboxy groups (Fig. 4c, e), except for the case that the all phenylene groups are in the orthogonal conformation [74]. Meanwhile, the PhT motif possessing three frustrated dimers (Fig. 4b) has not been observed so far. The frustrated dimer is sometimes trapped by a polar molecule used as a solvent for crystallization, such as DMF, and fractured to form the “truncated catemer” type dimer, to release the frustration (Fig. 4d,f) [91]. This structural changes caused by the solvent insertion can change stacking manners of the H-NexNets as observed in the case of T18 [91].
Structural features of PhT motif. a Cooperative incline of the peripheral phenylene groups. b PhT with three frustrated dimers. c PhT with one frustrated dimer. d PhT with one fractured dimer by insertion of a DMF molecule. Crystal structures of e frustrated and f fractured PhT observed in crystals of T18 [91]
HOFs composed of planar π-conjugated hydrocarbons
Firstly, four kinds of rigid and planar C3PIs with sides of two different lengths (Tp, T12, T18, and Ex) were designed, synthesized, and subjected to HOF construction [93]. Such C3PIs were expected to form a 2D H-HexNet with multi void spaces [94]. Moreover, the size and shape of the void can be varied by changing the side length of the C3PIs. Construction of LA-H-HexNets were performed by simple recrystallization, where a mixed solution of a highly polar solvent such as DMF and a high-boiling aromatic solvent such as 1,2,4-trichlorobenzene (124TCB) or methyl benzoate (MeBz) was slowly evaporated at relatively high temperature (50–120 °C) over a couple days to yield single crystals suitable for X-ray diffraction analysis. Note that recrystallization at relatively low temperature such as at 30 °C results in concomitant formation of incompletely-networked crystals, in which one or more carboxy groups form H-bonds with solvent molecules, preventing formation of completely-networked LA-H-HexNet structures. At this moment, the author suggests that selective formation of the networked structures at high temperature is due to the following entropy compensation effect. Namely, formation of a completely-networked structure is entropically less favorable because abundant DMF molecules should be excluded during crystallization. To accomplish the networked structure, high temperature is required to compensate entropical disadvantage.
Crystal structures of LA-H-HexNets crystals of Tp, T12, T18, and Ex are shown in Fig. 5. It is noteworthy that these four C3PIs form H-HexNets in the same way as expected. In the H-HexNet sheet, three kinds of pores (Voids I, II, and III) are formed. Void I is a pore within the PhT motif. Void II is the largest pore, whose dimension depends on the size of the C3PI cores: the longer sides of void II are the same (15.8 Å) in the four systems, while the shorter sides ranged from 2.0 to 11.4 Å. Void III is the inherent pore located at the center of the cyclic compounds T12, T18, and Ex. The H-HexNet sheets stack without interpenetration to give porous LA-H-HexNets (named as Tp-1, T12-1, T18-1, and Ex-1, respectively). Although the molecules give isostructural H-HexNets sheets, their stacking manners are not the same but depend on the molecular structures. The neighbored H-HexNet layers are stacked by weak interactions such as face-to-face (π/π), face-to-edge (CH/π), and CH/O interactions. The void ratio of the frameworks calculated by PLATON software are 54%, 41%, 58%, and 59%, respectively, and solvent molecules used for recrystallization are accommodated in the voids until activation.
In the case of Tp, with the smallest π-conjugated core among them, at least four polymorphs of LA-H-HexNets including Tp-1 are formed [95]. This is probably due to nonspecific weak interlayer interactions, as well as rotational flexibility of the peripheral phenylene rings. Other Tp derivatives, which have two methyl and fluoro groups at the ortho-positions of the carboxy group (TpMe and TpF, respectively) were also applied for HOF construction to investigate steric and conformational effects around hydrogen bonding moieties [96]. Theoretical calculation indicates that substituent groups have no effects on the binding energy of H-bonded dimerization, which is approximately 15 kcal mol− 1 and that both carboxy and phenylene groups exhibit twisted conformations. Experimentally, TpMe yielded at least three polymorphic LA-H-HexNets with versatile conformation of the peripheral carboxy and phenylene moieties. TpF gave one crystalline form possessing LA-H-HexNet structure, in which the peripheral groups are significantly disordered.
Activation of the LA-H-HexNets was conducted by immersing the as-formed crystalline powder into benzene for overnight at room temperature, followed by laying it under vacuum condition (0.2 kPa) for 1 day at 100 °C to give completely-desolvated materials (Tp-apo, T12-apo, T18-apo, and Ex-apo). The materials still exhibit obvious PXRD patterns. However, they are not in agreement with the original patterns of the solvate crystals, indicating that crystal structures of the LA-H-HexNets changed during activation processes. Therefore, crystal structures of the activated forms were attempted to be identified based on PXRD patterns, and were successfully obtained the reasonable structures in the cases of Tp-apo and T12-apo by using the crystal structure prediction (CSP) technique and powder X-ray analysis including Rietveld refinement, respectively. Structural analysis of Tp-apo based on the experimental PXRD data was failed. Alternately, we applied the classic CSP technique to estimate the structure. Given P-1 crystal with Z’ = 1, approximately 5500 crystal structures were generated by Monte Carlo calculations with a geometrically optimized molecular model of Tp, and subsequently optimized by the force field. PXRD patterns of the generated structures and experimental Tp-apo were carefully compared, and one candidate structure (CDFF), which is pointed by the red arrow in the lattice energy-density plot of the generated structures, was picked up [Fig. 6a (top)]. The structure of CDFF was further optimized by DFT calculation at the vdW-DF2 level with the Quantum Espresso program [97]. The parameters of the optimized structure (CDDFT) are estimated to be a = 22.52 Å, b = 22.52 Å, c = 7.14 Å, α = 105.05°, β = 78.90°, γ = 59.99°, and V = 2900.87 Å3. The CDDFT nicely reproduces the experimental PXRD pattern of Tp-apo in the low-angle region [Fig. 6a (bottom)]. In the structure of Tp-apo, the H-HexNet is retained, while stacking manner of H-HexNets is changed so as to accomplish larger overlap between the adjacent layers (Fig. 6c). Triangular channels with a diameter of ca. 8.5 Å run along the c axis. The void ratio of the pore is 33%. Crystal structure of T12-apo was, on the other hand, successfully solved by the Rietveld refinement based on the experimental PXRD pattern with the following parameters [a = 20.13(2) Å, b = 43.36(4) Å, c = 7.401(7) Å, β = 90.509(4)°, Rp = 5.73%, Rwp = 8.00%] (Fig. 6b). The analysis indicates that T12-apo also retains a layered structure of H-HexNet sheets, although H-bonded dimers of the carboxyphenyl groups are deformed (Fig. 6d). The stacking manner of the layers are also slightly altered, and one-dimensional pores with a triangular aperture with width of 8.8 Å are formed along the c axis. A small spherical room with a diameter of 2.8 Å is branched from the main pore. The void ratio of T12-apo is 38%.
Regarding stability of HOFs, VT-PXRD experiment reveals that frameworks of Tp-apo, T12-apo, T18-apo, and Ex-apo decompose at 323 °C, over 360 °C, 242 °C, and 249 °C, respectively. On the other hand, HOFs composed of TpF and TpMe exhibit relatively lower decomposition temperatures (301 °C and 155 °C, respectively) compared with Tp.
Tp-apo and T12-apo show a type-I N2 sorption isotherm at 77 K. Similarly, Tp-apo has a type-I CO2 sorption isotherm, while T12-apo exhibit a two-stepped sorption isotherm with hysteric behavior for CO2 at 195 K. Calculated SA(BET) values of Tp-apo and T12-apo based on CO2 sorption are to be 788 m2g− 1 and 557 m2g− 1, respectively. Although T18-apo and Ex-apo also absorb N2 and CO2, the details are not shown because of their ambiguous structures. Tp-apo and T12-apo were also demonstrated to absorb volatile hydrocabons such as ethane, n-butane, and n-hexane. Tp-apo shows similar amounts of uptake toward n-hexane and cyclohexane, while T12-apo shows better absorption property for cyclohexane than for n-hexane, probably due to subtle valance of their size matching that maximizes attractive van der Waals interactions [98].
It is noteworthy that only T12-apo shows the stepwise CO2 sorption behavior with hysteresis at 195 K (Fig. 7a). Generally, such hysteresis is recognized to be generated from (1) capillary condensation taking place in mesopores or (2) framework flexibility and the existence of molecular gates. To reveal the origin of the sorption behavior, in-situ PXRD measurements were performed, disclosing that T12-apo transforms among four kinds of crystalline forms (states 1–4) reversibly during CO2 absorption-desorption process at 195 K (Fig. 7b) [99]. Although the precise crystal structures have not been determined, the observed PXRD patterns indicated that the layered structure of T12-apo changes by distorting the network, slipping of the H-HexNet layers, and/or increasing in the interlayer distance (States 1–4 shown in Fig. 7c). These results indicate that organic layered crystals is much more flexible than our conventional consideration and the results can aid the construction of soft porous crystalline materials.
CO2 sorption-induced structural changes of T12-apo at 195 K. a Sorption isotherms. b in situ PXRD patterns, each of which is recorded at the corresponding pressure (a–o) in the isotherm (a). In the isotherms, solid and open symbols denote absorption and desorption processes, respectively, and the pressure that the PXRD pattern was recorded at is shown in the parentheses. c Proposed structural changes including slippage, expansion, and deformation of the layered structure
The H-bonded low density framework can be applied not only for gas and hydrocarbon absorbents but for a platform to achieve unusual arrangement of fictional molecules. Crystallization of T18 in the presence of fullerene C60 yields two types of inclusion crystals T18-C60-1 and -2, in which T18 forms LA-H-HexNets and C60 molecules are included in void space of the frameworks (Fig. 8a,b). Interestingly, void II in T18-C60-2 accommodates two C60 molecules at its corners and the resulting dimeric array of C60 is aligned within the H-HexNet framework in a way isolated from the adjacent dimers (Fig. 8c). The distance between the centeroids of the nearest two C60 molecules (A and B) is 11.2 Å and that between the second nearest two molecules is 15.1 Å. The isolated C60 pair observed in the present system is a unique type of C60 array [100]. Furthermore, LA-H-HexNets composed of π-conjugate molecules, particularly T12, were revealed to exhibit significant fluorescence emission [101, 102]. The results imply that the LA-H-HexNet can be a useful platform to align functional molecules, aiming to developing optoelectronic functional materials.
Bowl-shaped C3PI: a sumanene derivative
As described in the former sections, C3PIs with a planar flat core, such as triphenylene derivative (Tp), form planar 2D H-HexNets sheets, which stack without interpenetration to yield porous layered frameworks [95]. Non-planar bowl-shaped C3PIs, on the other hand, seem to be difficult to form the same a planar 2D H-HexNets. The author became interested in a periodic H-bonded framework composed of bowl-shaped π-conjugated molecules, although a handful examples of MOFs based on corannulene and sumanene were reported [103,104,105]. Such structure can provide useful information how curved molecules accomplish fully H-bonded, networked structures. Furthermore, unique physical properties originating from the curved π-systems are also expected. From these aspect, C3PI possessing buckybowl core (i.e. sumanene) [106] CPSM was synthesized and subjected to network formation experiments [107].
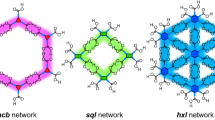
CPSM gives three types of crystals, and interestingly two of them are revealed to form fully H-bonded networked structures (Fig. 9). In crystal CPSM-1, the molecules are connected via the PhT motif as in the case of Tp-1, to give a H-HexNet sheet. The sheet is undulated with a periodicity of 35.3 Å due to an alternate alignment of bowl-up and bowl-down buckybowls. To satisfy H-bond formation, two carboxyphenyl groups bend outward and the bowl becomes shallower compared with the pristine smanene: The bowl depth (BD) is 0.985 Å for CPSM-1 and 1.11 Å for pristine smanens) [108, 109]. In crystal CPSM-2, the molecules are forms a hamburger-shaped dimer by interdigitating peripheral phenylene groups. The dimer has internal void with volume of 140 Å3, in which one DMF molecule is encapsulated. The dimers are connected through a trefoil knot-shaped H-bonding motif to form a bilayer. Since all carboxy groups of the molecule are capable of forming H-bonds without severe geometrical frustration, the BD value in CPSM-2 is 1.15 Å, which is close to that of the pristine sumanene. In connection with network topology, CPSM-1 has 6-connected a two-dimensional uninodal hxl net. CPSM-2 also has a 6-connected two-dimensional network, while the topology of the network has been hitherto unknown (Fig. 9c,f).
Since a networked layer in CPSM-1 and -2 crystals has less contact with the neighboring layers due to a bumpy surface, the crystals are expected to be deformed by compression. To confirm this point, the single crystals of CPSM-1 and CPSM-2, placed respectively in a diamond anvil cell, were subjected to SXRD analysis under high pressure conditions. CPSM-1 immediately loses its crystallinity upon adding pressure, while CPSM-2 keeps single crystallinity, and its cell parameters show significant anisotropic changes upon addition of isotropic hydrostatic fluid pressure. Mainly, only the c axis is shortened by 11.0% at 970 MPa compared with that under ambient pressure. This transformation is irreversible. SXRD analysis at 970 MPa reveals that two contacted CPSM molecules (A and B) are slipped along their curved surfaces as shown in Fig. 10, resulting in shrinkage of interlayer distance. Such a dynamic behavior between non-planar 2D sheets is hitherto unknown and can provide new insight into 2D-networked architectures based on non-planar π-conjugated systems.
Pressure-induced anisotropic structural changes observed in CPSM-2. a Changes in the unit cell upon increasing pressure. b Molecular packing under amnient pressure at − 120 °C. c Molecular packing under 970 MPa at 20 °C. In graph (a), open symbols refer the cell parameters obtained from SXRD analysis conducted at − 120 °C under ambient pressure corresponding the crystal (b). Closed symbols refer those obtained from SXRD analysis conducted at ambient temperature with changing pressure
Nitrogen-incorporated C3PIs: a hexaazatri-phenylene and -naphthylene derivatives
In the following parts, the author describes HOFs constructed by N-contained π-conjugated molecules. Introducing nitrogen atoms into π-conjugated polycyclic aromatic systems is capable of altering frontier orbital levels and interacting and/or coordinating to cationic species such as metal cations and proton. Therefore, HOFs composed of such systems are expected to show not only permanent porosity but also other functionalities such as optoelectronic properties and external stimuli-responsiveness. In order to construct such multi-functional HOFs, two kinds of π-conjugated cores, hexaazatriphenylene (HAT) and hexaazatrinaphthylene (HATN) [110], were applied. Firstly, a HAT derivative possessing six carboxyphenyl groups (CPHAT) was synthesized and subjected to HOF construction. CPHAT is a closely resembling analogue of Tp which gives layered HOF (LA-H-HexNet). However, surprisingly, CPHAT gives no layered HOF (LA-H-HexNet) but a 3D networked HOF (CPHAT-1) [92]. In solid state, a HAT core is not flat but has a propeller-like twisted conformation due to packing force. Owing to the conformation, the peripheral six carboxyphenyl groups are alternately directed up and down, resulting in 3D H-bonded network with primitive cubic (pcu) topology. The network is then interpenetrated by fourfold to yield the whole crystal structure (Figs. 3b, 11). It is remarkable that the framework of CPHAT-1 is much more stable than that of Tp: the activated framework CPHAT-1a has retained single crystallinity, capable of being solved by SXRD analysis, and moreover, is thermally stable over 300 °C and has durability against polar solvents, as described later. This significant stability is provided not only by interpenetration of 3D network but by shape-fitted tight docking among the propeller-shaped π-conjugated cores. Then, keeping this in the mind, the author hypothesized that “shape-fitted docking” [111] of HAT cores can play an important role to accomplish highly-stable isostructural HOFs, and explored other HAT derivatives which possess HAT core as a common platform and H-bonding arms with different length and structures.
To date, two HAT-based HOFs (CPHAT-1a and CBPHAT-1a), which have phenylene and biphenylene arms, were reported [92, 112]. A preliminary crystal structure of one solvated HOF composed of a HAT derivative possessing diphenylacetylene arms (CTolHAT-1) was also revealed by the author’s group as shown in Fig. 11c, although the details have not published yet. These HOFs have the closely same hierarchical structures from molecular conformation, H-bonding motif, and the interpenetrated 3D pcu-frameworks. On the other hand, their periodic distances (21.6 Å, 29.8 Å, and 34.3 Å, respectively) and number of networks interpenetrated (4, 6, and 8, respectively) depends on the length of the arms of the C3PIs.
CPHAT and CBPHAT were crystallized by slow evaporation of a DMF and 124TCB mixed solution at 100 °C and 60 °C, respectively, to give the P-3 and R-3 framework crystals including 124TCB molecules [CPHAT-1(124TCB) and CBPHAT-1(124TCB), respectively]. Activation of CPHAT-1(124TCB) and CBPHAT-1(124TCB) crystals at 200 °C and 150 °C, respectively, under vacuum conditions gives empty crystals CPHAT-1a and CBPHAT-1a with permanent porosity. It is remarkable that they retain their original frameworks and single crystallinity, which enabled to perform SXRD analysis to reveal the structures. CPHAT-1a and CBPHAT-1a have thermal stability to keep the framework up to 339 °C and 305 °C, respectively, and also have solvent durability against polar mediates such as water, alcohol, and hydrochloric acid. To evaluate their porosity, the activated HOFs were subjected to gas sorption experiments as shown in Fig. 12. Due to larger size of the channel (widths of aperture are 14.5 Å for CBPHAT-1a and 6.7 Å for CPHAT-1a), CBPHAT-1a shows much amount of uptake compared with CBPHAT-1a. Moreover, it is noteworthy that only CPHAT-1a with narrower channles shows no absorption toward N2, but gives type-I sorption isotherm toward CO2, even the kinetic diameters of both N2 and CO2 are smaller than width of the aperture. Generally, CO2 tends to be more absorbed than N2 in the case of porous materials composed of organic molecules because organic groups such as aromatic benzene rings or carbonyl groups can interact with a quadrupolar CO2 more than nonpolar N2. In the case of CPHAT-1a, however, the origin of the CO2 selectivity seems not to be simple, and further investigation is required. SA(BET) values are estimated to be 649 m2g− 1 for CPHAT-1a based on the CO2 absorption isotherm and to be 1288 m2g− 1 for CBPHAT-1a based on the N2 absorption isotherm.
As described above, single crystals of empty CPHAT-1a were successfully obtained, which encouraged us to demonstrate anisotropic sorption behaviors of 1D porous channels by using the single crystals. As shown in Fig. 13a, a gradual penetration of iodine into the crystal of CPHAT-1a is observed when the crystals is placed under iodine vapor at ambient condition. An edge of the single crystal is immediately colored with red, and the colored region is extended and reaches to the other edge within 10 min, although the iodine penetration behavior highly depends on crystalline sizes, shapes, and conditions (e.g. existence of cracks). When the iodine vapor is removed, the dark-red crystal is gradually discolored in relatively longer period of time compared with the absorption process. An alignment of iodine within the channel of CPHAT-1a was determined by SXRD analysis of the iodine-filled crystal (Fig. 13b,c). CPHAT-1a retains its original framework and iodine molecules are included in the channels. Iodine molecules are aligned in a threefold helical fashion, and each of iodine molecules contacts with the neighboring ones with a nearly perpendicular manner: the intermolecular distance and contact angle between the neighboring iodine molecules (A and B) are 3.60 Å and 82.8°, respectively, which are typical for the perpendicular halogen-bond in a crystalline state [113]. Moreover, the iodine molecule forms a halogen-bond with the oxygen atom of the carboxy group on the channel surface (distance of O⋯I is 3.08 Å, angles of I–I⋯O and C–O⋯I are 150.4° and 144.5°, respectively) [114]. Because of this attractive interactions, iodine molecules prefer to be accommodate in the channel and the occupancy of total iodine molecules is estimated by crystallographic analysis to be 79%.
Iodine sorption behavior of a CPHAT-1a single crystal. a Sorption and desorption behavior of a single crystal. Scale bar: 100 µm. b Crystal structure of iodine-included CPHAT-1a and iodine alignment, where d and θ the intermolecular distance and contact angle between the neighboring iodine molecules, respectively. c Interaction between the channel wall and iodine molecules. Iodine molecules are disordered into two sites (I and II) with an abundance ratio of 0.61 and 0.39, respectively, although for clarity, only the major population is shown in b
N-contained π-core was further expanded from HAT to HATN. A derivative CPHATN was synthesized and crystallized by slow evaporation of a mixed solvent of N-methyl pyrodidone (NMP) and 124TCB at 100 °C to yield a 124TCB solvate crystal with a H-bonded networked structure [CPHATN-1(124TCB)] [115]. Contrary to CPHAT, CPHATN forms the PhT motif via H-bonding of carboxy groups to give a H-HexNet sheet, which then stacks without interpenetration to give a LA-H-HexNet CPHATN-1(124TCB). Larger π-conjugated core of CPHATN may prevent the core twisting to form a 3D network, which is observed in the HAT-systems. Moreover, it is noteworthy that carboxy groups do not make H-bonds with the basic pyrazine moieties, which remain “vacant” and play a role to show acid responsiveness.
To explore activation condition of the material, TG-DTA and VT-PXRD experiments of as-formed crystalline bulk of CPHATN-1(124TCB) were undertaken. TG curve indicates that the material is completely desolvated at ca. 220 °C to give empty framework CPHATN-1a. Moreover, VT-PXRD changes show that the peaks at 4.30, 5.18, 8.62, and 10.48° starts to appear at 83 °C and slightly shifts to 4.34, 5.24, 8.74, 10.52° up to 114 °C, indicating shrinkage of the crystallographic cell (Fig. 14a), although the initial patterns at around room temperature are not clear as in the case of other systems [93]. The peak intensity increases as heating, reaches plateau at ca. 250 °C. The intensity remains up to 360 °C, which is the limitation temperature of the equipment, indicating that the framework of CPHATN-1a has significant thermal stability enough to retain the porous structure at high temperature.
Based on the results mentioned above, activation of CPHATN-1(124TCB) was completed by heating at 190 °C for 3 days under a vacuum condition to yield CPHATN-1a, which again partly retained single crystalline domain suitable for SXRD analysis (Fig. 14b). CPHATN-1a has a slightly different LA-H-HexNet structure with CPHATN-1(124TCB). Although stacking way of the molecules are quite similar, the conformationally-frustrated carboxy dimer is deformed upon removal of the solvent molecules to transform the framework and a ratio of void volume calculated by PLATON software is decreased from 26 to 20%. 1D channels with an aperture width of ca. 6.2 Å are survived after activation. CPHATN-1a is less stable toward polar solvent compared with 3D networked CPHAT-1a and CBPHAT-1a, due to its layered 2D network. Gas sorption experiments of CPHATN-1a give SABET estimated to be 379 m2g− 1 based on N2 sorption isotherm at 77 K.
a VT-PXRD pattern changes of as-formed solvate CPHATN-1(124TCB) upon heating from room temperature to 360 °C. Black arrow refers slight shrinkage of the cell upon removal of 124TCB molecules. b Crystal structures of activated HOF CPHATN-1a (inset: visualized surface of the pore). Structural motifs of c CPHATN-1(124TCB) and d CPHATN-1a, where the conformationally frustrated carboxylic dimer is distorted upon activation as pointed by arrows
Interestingly, CPHATN-1a changes its color from yellow to reddish-brown and its fluorescence is switched OFF when exposed to both 37%-HCl aqueous solution and HCl vapor (Fig. 15a). While the original yellow color and fluorescence intensity are then recovered upon removal of HCl by heating the materials or leaving them under an ambient condition for a couple of ten minutes. These process are reversible and repeatable. Upon exposing to HCl, a new absorption band at 500–600 nm appears in UV–Vis spectrum and the emission band at 539 nm is strongly quenched in fluorescence spectrum (Fig. 15c,d). These observations clearly indicate the sensitivity of CPHATN-1a to HCl, and interactions between protons and weakly-basic pyrazine moieties of HATN core. Removal of HCl from CPHATN-1a resulted in recover of the original absorption and emission spectra. This is, to our knowledge, the first example of HOFs with external-stimuli responsiveness in color and emission. Furthermore, HCl exposure experiments demonstrate that not only CPHATN-1a but CBPHAT-1a also show HCl responsiveness [116]. The HCl responsiveness of CBPHAT-1a is also reversible (Fig. 15b). However, CBPHAT-1a shows red shift of the fluorescence band from 489 nm, via 603 nm after 5 min, and to 675 nm at 40 min, as well as the clear new absorption band at ca. 600 nm (Fig. 15e,f). The observed successive shift on the fluorescence spectra is explained in terms of multi-step protonation of the nitrogen atoms of the CBPHAT core when sensing the HCl atmosphere. The present results would open a door for a new porous organic materials with stimuli responsiveness.
HCl responsiveness of CPHATN-1a and CBPHAT-1a. Reversible color changes of crystalline bulks of a CPHATN-1a and b CBPHAT-1a upon exposure to and removal of HCl. c Absorption and d emission spectral changes of CPHATN-1a crystalline bulk upon exposure to HCl atmosphere for 40 min and after removing HCl by leaving in air for 48 h. Gradual changes of e absorption and f emission spectra of the CBPHAT-1a crystalline bulk upon up on exposure to HCl for 5 min and 40 min
Conclusion
Conventionally, a hydrogen bond have been regarded as too weak interaction to be used for construction of molecular crystals with self-supported large pores. However, recent advance in the HOF chemistry enable it getting much easier to construct porous frameworks with permanent porosity. To achieve construction of designed frameworks, H-bonding moieties that allow highly directional H-bonds with predictable manners and rigid molecular skeletons exclusive degree of conformational freedom are required. Furthermore, to realize rigid framework with permanent porosity, it is necessary to introduce secondary interacting moieties capable of well-fitted intermolecular contacts to support the H-bonded networks, in addition to applying geometrically well-preorganized supramolecular synthons.
In connection with this, the author has demonstrated that C3-symmetric π-conjugated molecules (C3PIs) possessing three o-bis(4-carboxyphenyl)aryl moieties in the periphery can be good building blocks for systematic construction of various HOFs. Planar C3PIs give LA-H-HexNets structures. Thanks to the triangular H-bonded motif named phenylene triangle (PhT), C3PIs can form isostructural H-HexNet sheets, which stack via interlayer interaction such as π/π interaction to give layered HOFs. Non-planar C3PIs give 3D-networked rigid HOFs. C3PIs possessing HAT core form 1D π-stacked column via shape-fitted docking, to make the framework more rigid. Interpenetration of H-bonded networks also contributes to form rigid HOFs. It is also interesting that even bowl-shaped C3PI form H-bonded networked structures with hitherto-unknown network topology, in which all of carboxy groups satisfied H-bonded dimer formation. These results indicate that HOFs are one of the promising candidates for porous functional materials.
References
Slater, A.G., Cooper, A.I.: Function-led design of new porous materials. Science 348, aaa8075 (2015)
Yaghi, O.M., Li, G., Li, H.: Selective binding and removal of guests in a microporous metal-organic framework. Nature 378, 703–706 (1995)
Kondo, M., Fujimoto, K., Ohkubi, T., Asami, A., Noro, S., Kitagawa, S., Ishii, T., Matsuzaki, H.: Novel extended linear structure of decavanadate anions linked by bis(4-pyridinium) disulfide (H2dpds), {{(H2dpds)2[V10O26(OH)2]·10H2O}}. Chem. Lett. 28, 291–292 (1999)
Li, J.-R., Sculley, J., Zhou, H.-C.: Metal-organic frameworks for separation. Chem. Rev. 112, 869–932 (2012)
Cook, T.R., Zheng, Y.-R., Stang, P.J.: Metal-organic frameworks and self-assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 113, 734–777 (2013)
Li, M., Li, D., O’Keeffe, M., Yaghi, O.M.: Topological analysis of metal-organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle. Chem. Rev. 114, 1343–1370 (2014)
Côté, A.P., Benin, A.I., Ockwig, N.W., O’Keeffe, M., Matzger, A.J., Yaghi, O.M.: Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005)
Waller, P.J., Gándara, F., Yaghi, O.M.: Chemistry of covalent organic frameworks. Acc. Chem. Res. 48, 3053–3063 (2015)
Feng, X., Ding, X., Jiang, D.: Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012)
Ding, S.-Y., Wang, W.: Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013)
Dogru, M., Bein, T.: On the road towards electroactive covalent organic frameworks. Chem. Commun. 50, 5531–5546 (2014)
Zhao, W., Xia, L., Liu, X.: Covalent organic frameworks (COFs): perspectives of industrialization. CrystEngComm 20, 1613–1634 (2018)
Barbour, L.J.: Crystal porosity and the burden of proof. Chem. Commun. 11, 1163–1168 (2006)
McKeown, N.B.: Nanoporous molecular crystal. J. Mater. Chem. 10, 10588–10597 (2010)
Holst, J.R., Trewin, A., Cooper, A.I.: Porous organic molecules. Nat. Chem. 2, 915–920 (2010)
Tian, J., Thallapally, P.K., McGrail, B.P.: Porous organic molecular materials. CrystEngComm 14, 1909–1919 (2012)
Mastalerz, M.: Permanent porous materials from discrete organic molecules—towards ultra-high surface areas. Chem. Eur. J. 18, 10082–10091 (2012)
Cooper, A.I.: Porous molecular solids and liquids. ACS Cent. Sci. 3, 544–553 (2017)
Barrer, R.M., Shanson, V.H.: Dianin’s compound as a zeolitic sorbent. J. Chem. Soc. Chem. Commun. 9, 333–334 (1976)
Sozzani, P., Comotti, A., Simonutti, R., Meersmann, T., Logan, J.W., Pines, A.: A porous crystalline molecular solid explored by hyperpolarized xenon. Angew. Chem. Int. Ed. 39, 2695–2699 (2000)
Sozzani, P., Bracco, S., Comotti, A., Ferretti, L., Simonutti, R.: Methane and carbon dioxide storage in a porous van der Waals crystal. Angw Chem. Int. Ed. 44, 1816–1820 (2005)
Simard, M., Su, D., Wuest, J.D.: Use of hydrogen bonds to control molecular aggregation. self-assembly of three-dimensional networks with large chambers. J. Am. Chem. Soc. 113, 4696–4698 (1991)
Shimizu, L.S., Hughes, A.D., Smith, M.D., Davis, M.J., Zhang, B.P., Loye, H.-C., Shimizu, K.D.: Self-assembled nanotubes that reversibly bind acetic acid guests. J. Am. Chem. Soc. 125, 14972–14973 (2003)
Brunet, P., Simard, M., Wuest, J.D.: Molecular tectonics. porous hydrogen-bonded networks with unprecedented structural integrity. J. Am. Chem. Soc. 119, 2737–2738 (1997)
Palmans, A.R.A., Vekemans, J.A.J.M., Kooijman, H., Spek, A.L., Meijer, E.W.: Hydrogen-bonded porous solid derived from trimesic amide. Chem. Commun. 22, 2247–2248 (1997)
Saha, B.K., Jetti, R.K.R., Reddy, L.S., Aitipamula, S., Nangia, A.: Halogen trimer-mediated hexagonal host framework of 2,4,6-tris(4-halophenoxy)-1,3,5-triazine. Supramolecular isomerism from hexagonal channel (X = Cl, Br) to cage structure (X = I). Cryst. Growth Des. 5, 887–899 (2005)
Maly, K.E., Gagnon, E., Maris, T., Wuest, J.D.: Engineering hydrogen-bonded molecular crystals built from derivatives of hexaphenylbenzene and related compounds. J. Am. Chem. Soc. 129, 4306–4322 (2007)
Ogoshi, T., Yamagishi, T., Nakamoto, Y.: Pillar-shaped macrocyclic hosts pillar[n]arenes: new key players for supramolecular chemistry. Chem. Rev. 116, 7937–8002 (2016)
Sakamoto, H., Fujimori, T., Li, X., Kaneko, K., Kan, K., Ozaki, N., Hijikata, Y., Irle, S., Itami, K.: Cycloparaphenylene as a molecular porous carbon solid with uniform pores exhibiting adsorption-induced softness. Chem. Sci. 7, 4204–4210 (2016)
Atwood JL, Davies JED, MacNicol DD (eds) (1984) Inclusion Compound, vol 1–3. Academic Press, London
Endo, K., Sawaki, T., Koyanagi, M., Kobayashi, K., Masuda, H., Aoyama, Y.: Guest-binding properties of organic crystals having an extensive hydrogen-bonded network: an orthogonal anthracene-bis(resorcinol) derivative as a functional organic analog of zeolites. J. Am. Chem. Soc. 117, 8341–8352 (1995)
Miyata, M., Shibakami, M., Chirachanchai, S., Takemoto, K., Kasai, N., Miki, K.: Guest-responsive structural changes in cholic acid intercalation crystals. Nature 343, 446–447 (1990)
He, Y., Xiang, S., Chen, B.: A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 133, 14570–14573 (2011)
Yang, W., Greenaway, A., Lin, X., Matsuda, R., Blake, A.J., Wilson, C., Lewis, W., Hubberstey, P., Kitagawa, S., Champness, N.R., Schröder, M.: Exceptional thermal stability in a supramolecular organic framework: porosity and gas storage. J. Am. Chem. Soc. 132, 14457–14469 (2010)
Tian, J., Zhou, T.-Y., Zhang, S.-C., Aloni, S., Altoe, M.V., Xie, S.-H., Wang, H., Zhang, D.-W., Zhao, X., Liu, Y., Li, Z.-T.: Three-dimensional periodic supramolecular organic framework ion sponge in water and microcrystals. Nat. Commun. 5, 5574 (2014)
Yamamoto, A., Uehara, S., Hamada, T., Miyata, M., Hisaki, I., Tohnai, N.: Diamondoid porous organic salts toward applicable strategy for construction of versatile porous structures. Cryst. Growth Des. 12, 4600–4606 (2012)
Han, Y.-F., Yuan, Y.-X., Wang, H.-B.: Porous hydrogen-bonded organic frameworks. Molecules 22, 266 (2017)
Luo, J., Wang, J.-W., Zhang, J.-H., Lai, S., Zhong, D.-C.: Hydrogen-bonded organic frameworks: design, structures and potential applications. CrystEngComm 20, 5884–5898 (2018)
Lu, J., Cao, R.: Porous organic molecular frameworks with extrinsic porosity: a platform for carbon storage and separation. Angew. Chem. Int. Ed. 55, 9474–9478 (2016)
Lin, R.-B., He, Y., Ki, P., Wang, H., Zhou, W., Chen, B.: Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 48, 1362–1389 (2019)
Hisaki, I., Chen, X., Takahashi, K., Nakamura, T.: Designing hydrogen-bonded organic frameworks (HOFs) with permanent porosity. Angew. Chem. Int. Ed. 58, 11160–11170 (2019)
Adachi, T., Ward, M.D.: Versatile and resilient hydrogen-bonded host frameworks. Acc. Chem. Res. 49, 2669–2679 (2016)
Pulido, A., Chen, L., Kaczorowski, T., Holden, D., Little, M.A., Chong, S.Y., Slater, B., McMahon, D.P., Bonillo, B., Stackhouse, C.J., Stephenson, A., Kane, C.M., Clowes, R., Hasell, T., Cooper, A.I., Day, G.M.: Functional materials discovery using energy-structure-function maps. Nature 543, 657–666 (2017)
Cui, P., McMahon, D.P., Spackman, P.R., Alston, B.M., Little, M.A., Day, G.M., Cooper, A.I.: Mining predicted crystal structure landscapes with high throughput crystallization: old molecules, new insights. Chem. Sci. 10, 9988–9997 (2019)
Maspoch, D., Domingo, N., Ruiz-Molina, D., Wurst, K., Tejada, J., Rovira, C., Veciana, J.: A robust nanocontainer based on a pure organic free radical. J. Am. Chem. Soc. 126, 730–731 (2004)
Yang, J., Dewal, M.B., Profeta, S., Smith Jr., M.D., Li, Y., Shimizu, L.S.: Origins of selectivity for the [2 + 2] cycloaddition of α,β-unsaturated ketones within a porous self-assembled organic Framework. J. Am. Chem. Soc. 130, 612–621 (2008)
Comotti, A., Bracco, S., Distefano, G., Sozzani, P.: Methane, carbon dioxide and hydrogen storage in nanoporous dipeptide-based materials. Chem. Commun. 3, 284–286 (2009)
Mastalerz, M., Oppel, I.: Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem. Int. Ed. 51, 5252–5255 (2012)
Luo, X.-Z., Jia, X.-J., Deng, J.-H., Zhong, J.-L., Liu, H.-J., Wang, K.-J., Zhong, D.-C.: A microporous hydrogen-bonded organic framework: exceptional stability and highly selective adsorption of gas and liquid. J. Am. Chem. Soc. 135, 11684–11687 (2013)
Lü, J., Perez-Krap, C., Suyetin, M., Alsmail, N.H., Yan, Y., Yang, S., Lewis, W., Bichoutskaia, E., Tang, C.C., Blake, A.J., Cao, R., Schröder, M.: A robust binary supramolecular organic framework (SOF) with high CO2 adsorption and selectivity. J. Am. Chem. Soc. 136, 12828–12831 (2014)
Chen, T.-H., Popov, I., Kaveevivitchai, W., Chuang, Y.-C., Chen, Y.-S., Daugulis, O., Jacobson, A.J., Miljanić, O.Š.: Thermally robust and porous noncovalent organic framework with high affinity for fluorocarbons and CFCs. Nat. Commun. 5, 5131 (2014)
Comotti, A., Bracco, S., Yamamoto, A., Beretta, M., Hirukawa, T., Tohnai, N., Miyata, M., Sozzani, P.: Engineering switchable rotors in molecular crystals with open porosity. J. Am. Chem. Soc. 136, 618–621 (2014)
Wang, H., Li, B., Wu, H., Hu, T.-L., Yao, Z., Zhou, W., Xiang, S., Chen, B.: A flexible microporous hydrogen-bonded organic framework for gas sorption and separation. J. Am. Chem. Soc. 137, 9963–9970 (2015)
Li, P., He, Y., Zhao, Y., Weng, L., Wang, H., Krishna, R., Wu, H., Zhou, W., O’Keeffe, M., Han, Y., Chen, B.: A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem. Int. Ed. 54, 574–577 (2015)
Yadav, V.N., Comotti, A., Sozzani, P., Bracco, S., Bonge-Hansen, T., Hennum, M., Görbitz, C.H.: Microporous molecular materials from dipeptides containing non-proteinogenic sesidues. Angew. Chem. Int. Ed. 54, 15684–15688 (2015)
Yang, W., Li, B., Wang, H., Alduhaish, O., Alfooty, K., Zayed, M.A., Li, P., Arman, H.D., Chen, B.: A microporous porphyrin-based hydrogen-bonded organic framework for gas separation. Cryst. Growth Des. 15, 2000–2004 (2015)
Nandi, S., Chakraborty, D., Vaidhyanathan, R.: A permanently porous single molecule H-bonded organic framework for selective CO2 capture. Chem. Commun. 52, 7249–7252 (2016)
Karmakar, A., Illathvalappil, R., Anothumakkool, B., Sen, A., Samanta, P., Desai, A.D., Kurungot, S., Ghosh, S.K.: Hydrogen-bonded organic frameworks (HOFs): a new class of porous crystalline proton-conducting materials. Angew. Chem. Int. Ed. 55, 10667–10671 (2016)
Yang, W., Wang, J., Wang, H., Bao, Z., Zhao, J.C.-G., Chen, B.: Highly interpenetrated robust microporous hydrogen-bonded organic framework for gas separation. Cryst. Growth Des. 17, 6132–6137 (2017)
Morshedi, M., Thomas, M., Tarzia, A., Doonan, C.J., White, N.G.: Supramolecular anion recognition in water: synthesis of hydrogen-bonded supramolecular frameworks. Chem. Sci. 8, 3019–3025 (2017)
Yan, Y., Yu, X., Yan, T., Wu, D., Ning, E., Qi, Y., Han, Y.-F., Li, Q.: A triptycene-based porous hydrogen-bonded organic framework for guest incorporation with tailored fitting. Chem. Commun. 53, 3677–3680 (2017)
Lin, Y., Jiang, X., Kim, S.T., Alahakoon, S.B., Hou, X., Zhang, Z., Thompson, C.M., Smaldone, R.A., Ke, C.: An elastic hydrogen-bonded cross-linked organic framework for effective iodine capture in water. J. Am. Chem. Soc. 139, 7172–7175 (2017)
Wang, H., Wu, H., Kan, J., Chang, G., Yao, Z., Li, B., Zhou, W., Xiang, S., Zhao, J.C.-G.: Chen. B: A microporous hydrogen-bonded organic framework with amine sites for selective recognition of small molecules. J. Mater. Chem. A 5, 8292–8296 (2017)
Luo, Y.-H., He, X.-T., Hong, D.-L., Chen, C., Chen, F.-H., Jiao, J., Zhai, L.-H., Guo, L.-H., Sun, B.-W.: A dynamic 3D hydrogen-bonded organic frameworks with highly water affinity. Adv. Funct. Mater. 28, 1804822 (2018)
Lü, J., Perez-Krap, C., Trousselet, F., Yan, Y., Alsmail, N.H., Karadeniz, B., Jacques, N.M., Lewis, W., Blake, A.J., Coudert, F.-X., Cao, R., Schröder, M.: Polycatenated 2D hydrogen-bonded binary supramolecular organic frameworks (SOFs) with enhanced gas adsorption and selectivity. Cryst. Growth Des. 18, 2555–2562 (2018)
Hashim, M.I., Le, H.T.M., Chen, T.-H., Chen, Y.-S., Daugulis, O., Hsu, C.-W., Jacobson, A.J., Kaveevivitchai, W., Liang, X., Makarenko, T., Miljanić, O.Š., Popovs, I., Tran, H.V., Wang, X., Wu, C.-H., Wu, J.I.: Dissecting porosity in molecular crystals: influence of geometry, hydrogen bonding, and [π···π] stacking on the solid-state packing of fluorinated aromatics. J. Am. Chem. Soc. 140, 6014–6026 (2018)
Han, B., Wang, H., Wang, C., Wu, H., Zhou, W., Chen, B., Jiang, J.: Postsynthetic metalation of a robust hydrogen-bonded organic framework for heterogeneous catalysis. J. Am. Chem. Soc. 141, 8737–8740 (2019)
Gong, W., Chu, D., Jiang, H., Chen, X., Cui, Y., Liu, Y.: Permanent porous hydrogen-bonded frameworks with two types of Brønsted acid sites for heterogeneous asymmetric catalysis. Nat. Commun. 10, 600 (2019)
Burnauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Ivasenko, O., Perepichka, D.F.: Mastering fundamentals of supramolecular design with carboxylic acids. common lessons from X-ray crystallography and scanning tunneling microscopy. Chem. Soc. Rev. 40, 191–206 (2011)
Desiraju, G.R.: Supramolecular synthons in crystal engineering—a new organic synthesis. Angew. Chem., Int. Ed. Engl. 34, 2311–2327 (1995)
Duchamp, D.J., Marsh, R.E.: The crystal structure of trimesic acid (benzene-1,3,5-tricarboxylic acid). Acta Crystallogr. B 25, 5–19 (1969)
Herbstein, F.H., Kapon, M., Reisner, G.M.: Catenated and non-catenated inclusion complexes of trimesic acid. J. Incl. Phenom. 5, 211–214 (1987)
Kobayashi, K., Shirasaka, T., Horn, E., Furukawa, N.: Two-dimensional hexagonal hydrogen-bonded network with triangle-like large cavities: hexakis(4-carboxyphenyl)benzene. Tetrahedron Lett. 41, 89–93 (2000)
Zentner, C.A., Lai, H.W.H., Greenfield, J.T., Wiscons, R.A., Zeller, M., Campana, C.F., Talu, O., FitzGerald, S.A., Rowsell, J.L.C.: High surface area and Z' in a thermally stable 8-fold polycatenated hydrogen-bonded framework. Chem. Commun. 51, 11642–11645 (2015)
Lai, H.W.H., Wiscons, R.A., Zentner, C.A., Zeller, M., Rowsell, J.L.C.: Supramolecular assembly of tris(4-carboxyphenyl)arenes: relationship between molecular structure and solid-state catenation motifs. Cryst. Growth Des. 16, 821–833 (2016)
Bassanetti, I., Bracco, S., Comotti, A., Negroni, M., Bezuidenhout, C., Canossa, S., Mazzeo, P.P., Marchió, L., Sozzani, P.: Flexible porous molecular materials responsive to CO2, CH2 and Xe stimuli. J. Mater. Chem. A 6, 14231–14239 (2018)
Hisaki, I., Affendy, N.Q.E., Tohnai, N.: Precise elucidations of stacking manners of hydrogen-bonded two-dimensional organic frameworks composed of X-shaped π-conjugated systems. CrystEngComm 19, 4892–4898 (2017)
Hu, F., Liu, C., Wu, M., Pang, J., Jiang, F., Yuan, D., Hong, M.: An ultrastable and easily regenerated hydrogen-bonded organic molecular framework with permanent porosity. Angew. Chem. Int. Ed. 56, 2101–2104 (2017)
Yin, Q., Zhao, P., Sa, R.-J., Chen, G.-C., Lü, J., Liu, T.-F., Cao, R.: An ultra-robust and crystalline redeemable hydrogen-bonded organic framework for synergistic chemo-photodynamic therapy. Angew. Chem. Int. Ed. 57, 7691–7696 (2018)
Zhou, Y., Liu, B., Sun, X., Li, J., Li, G., Huo, Q., Liu, Y.: Self-assembly of homochiral porous supramolecular organic frameworks with significant CO2 capture and CO2/N2 selectivity. Cryst. Growth Des. 17, 6653–6659 (2017)
Takeda, T., Ozawa, M., Akutagawa, T.: Jumping crystal of a hydrogen-bonded organic framework induced by the collective molecular motion of a twisted π system. Angew. Chem. Int. Ed. 58, 10345–10352 (2019)
Li, P., Li, P., Ryder, M.R., Liu, Z., Stern, C.L., Farha, O.K., Stoddart, J.F.: Interpenetration isomerism in triptycene-based hydrogen-bonded organic frameworks. Angew. Chem. Int. Ed. 58, 1664–1669 (2019)
Li, P., Chen, Z., Ryder, M.R., Stern, C.L., Guo, Q.-H., Wang, X., Farha, O.K., Stoddart, J.F.: Assembly of a porous supramolecular polyknot from rigid trigonal prismatic building blocks. J. Am. Chem. Soc. 141, 12998–13002 (2019)
Miyaura, N., Suzuki, A.: Palladium-catalyzed cross-coupling reactions of organobrom compounds. Chem. Rev. 95, 2457–2483 (1995)
Hisaki, I., Sakamoto, Y., Shigemitsu, H., Tohnai, N., Miyata, M., Seki, S., Saeki, A., Tagawa, S.: Superstructure-dependent optical and electrical properties of an unusual face-to-face, π-stacked, one-dimensional assembly of dehydrobenzo[12]annulene in the crystalline state. Chem. Eur. J. 14, 4178–4187 (2008)
Hisaki, I., Senga, H., Sakamoto, Y., Tsuzuki, S., Tohnai, N., Miyata, M.: Specific interaction between chloroform and the pockets of triangular annulene derivatives providing symmetry carry-over crystallization. Chem. Eur. J. 15, 13336–13340 (2009)
Hisaki, I., Osaka, K., Ikenaka, N., Saeki, A., Tohnai, N., Seki, S., Miyata, M.: Arrangement modulation of π-stacked columnar assemblies of octadehydrodibenzo[12]annulene: substituent effects of peripheral thienyl and phenyl rings. Cryst. Growth Des. 16, 714–721 (2016)
Hisaki, I., Manabe, N., Osaka, K., Saeki, A., Seki, S., Tohnai, N., Miyata, M.: Effects of ortho-phenyl substitution on molecular arrangements of octadehydrodibenzo[12]annulene. Bull. Chem. Soc. Jpn. 87, 323–333 (2014)
Hisaki, I., Senga, H., Shigemitsu, H., Tohnai, N., Miyata, M.: Construction of 1D π-stacked superstructures with inclusion channels through symmetry-decreasing crystallization of discotic molecules of C3 symmetry. Chem. Eur. J. 17, 14348–11453 (2011)
Hisaki, I., Nakagawa, S., Tohnai, N., Miyata, M.: A C3-symmetric macrocycle-based, hydrogen-bonded, multiporous hexagonal network as a motif of porous molecular crystals. Angew. Chem. Int. Ed. 54, 3008–3012 (2015)
Hisaki, I., Ikenaka, N., Gomez, E., Cohen, B., Tohnai, N., Douhal, A.: Hexaazatriphenylene-based hydrogen-bonded organic framework with permanent porosity and single-crystallinity. Chem. Eur. J. 23, 11611–11619 (2017)
Hisaki, I., Nakagawa, S., Ikenaka, N., Imamura, Y., Katouda, M., Tashiro, M., Tsuchida, H., Ogoshi, T., Sato, H., Tohnai, N., Miyata, M.: A series of layered assemblies of hydrogen-bonded, hexagonal networks of C3-symmetric π-conjugated molecules: a potential motif of porous organic materials. J. Am. Chem. Soc. 138, 6617–6628 (2016)
Dai, H., Wang, S., Hisaki, I., Nakagawa, S., Ikenaka, N., Deng, K., Xiao, X., Zeng, Q.: On-surface self-assembly of a C3-symmetric π-conjugated molecule family studied by STM: two-dimensional nanoporous frameworks. Chem. Asian J. 12, 2558–2564 (2017)
Hisaki, I., Ikenaka, N., Tohnai, N., Miyata, M.: Polymorphs of layered assemblies of hydrogen-bonded hexagonal networks caused by conformational frustration. Chem. Commun. 52, 300–303 (2016)
Hisaki, I., Ikenaka, N., Tsuzuki, S., Tohnai, N.: Sterically crowded hydrogen-bonded hexagonal network frameworks. Mater. Chem. Front. 2, 338–346 (2018)
Giannozzi, P., Baroni, S., Bonini, N., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Chiarotti, G.L., Cococcioni, M., Dabo, I., Dal Corso, A., Fabris, S., Fratesi, G., de Gironcoli, S., Gebauer, R., Gerstmann, U., Gougoussis, C., Kokalj, A., Lazzeri, M., Martin-Samos, L., Marzari, N., Mauri, F., Mazzarello, R., Paolini, S., Pasquarello, A., Paulatto, L., Sbraccia, C., Scandolo, S., Sclauzero, G., Seitsonen, A.P., Smogunov, A., Umari, P., Wentzcovitch, R.M.: QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 1–19 (2009)
Hisaki, I., Nakagawa, S., Suzuki, Y., Tohani, N.: CO2 sorption of layered hydrogen-bonded organic framework causes reversible structural changes involving four different crystalline states under ambient pressure. Chem. Lett. 47, 1143–1146 (2018)
Ogoshi, T., Saito, K., Sueto, R., Kojima, R., Hamada, Y., Akine, S., Moeljadi, A.M.P., Hirao, H., Kakuta, T., Yamagishi, T.: Separation of linear and branched alkanes using host-guest complexation of cyclic and branched alkane vapors by crystal state pillar[6]arene. Angew. Chem. Int. Ed. 57, 1592–1595 (2018)
Hisaki, I., Nakagawa, S., Sato, H., Tohnai, N.: Alignment of paired molecules of C60 within a hexagonal platform net-worked through hydrogen bonds. Chem. Commun. 52, 9781–9784 (2016)
Gomez, E., Gutierrez, M., Moreno, M., Hisaki, I., Nakagawa, S., Douhal, A.: Spectroscopy and dynamics of dehydrobenzo[12]annulene derivatives possessing peripheral carboxyphenyl groups: theory and experimental. Phys. Chem. Chem. Phys. 20, 7415–7427 (2018)
Gomez, E., Gutierrez, M., Cohen, B., Hisaki, I., Douhal, A.: Single crystal fluorescence behavior of a new HOF material: potential candidate for a new LED. J. Mater. Chem. C 6, 6929–6939 (2018)
Fellows, W.B., Rice, A.M., Williams, D.E., Dolgopolova, E.A., Vannucci, A.K., Pellechia, P.J., Smith, M.D., Krause, J.A., Shustova, N.B.: Redox-active corannulene buckybowls in a crystalline hybrid scaffold. Angew. Chem. Int. Ed. 55, 2195–2199 (2016)
Rice, A.M., Fellows, W.B., Dolgopolova, E.A., Greytak, A.B., Vavvucci, A.K., Smith, M.D., Karakalos, S.G., Krause, J.A., Avdoshenko, S.M., Popov, A.A., Shustova, N.B.: Hierarchical corannulene-based materials: energy transfer and solid-state photophysics. Angew. Chem. Int. Ed. 56, 4525–4529 (2017)
Yakiyama, Y., Hasegawa, T., Sakurai, H.: Formation of a large confined spherical space with a small aperture using flexible hexasubstituted sumanene. J. Am. Chem. Soc. 141, 18099–18103 (2019)
Sakurai, H., Daiko, T., Hirao, T.: A synthesis of sumanene, a fullerene fragment. Science 301, 1878 (2003)
Hisaki, I., Toda, H., Sato, H., Tohnai, N., Sakurai, H.: A hydrogen-bonded hexagonal buckybowl framework. Angew. Chem. Int. Ed. 56, 15294–15298 (2017)
Sakurai, H., Daiko, T., Sakane, H., Amaya, T., Hirao, T.: Structural elucidation of sumanene and generation of Its benzylic anions. J. Am. Chem. Soc. 127, 11580–11581 (2005)
Mebs, S., Weber, M., Luger, P., Schmidt, B.M., Sakurai, H., Higashibayashi, S., Onogi, S., Lentz, D.: Experimental electron density of sumanene, a bowl-shaped fullerene fragment; comparison with the related corannulene hydrocarbon. Org. Biomol. Chem. 10, 2218–2222 (2012)
Segura, J.L., Juárez, R., Ramos, M., Deoane, C.: Hexaazatriphenylene (HAT) derivatives: from synthesis to molecular design, self-organization and device applications. Chem. Soc. Rev. 44, 6850–6885 (2015)
Ascherl, L., Sick, T., Margraf, J.T., Lapidus, S.H., Calik, M., Hettstedt, C., Karaghiosoff, K., Döblinger, M., Clark, T., Chapman, K.W., Auras, F., Bein, T.: Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 8, 310–316 (2016)
Hisaki, I., Suzuki, Y., Gomez, E., Cohen, B., Tohnai, N., Douhal, A.: Docking strategy to construct thermostable, single-crystalline, hydrogen-bonded organic framework with high surface area. Angew. Chem. Int. Ed. 57, 12650–12655 (2018)
van Bolhuis, F., Koster, P.B., Migchelsen, T.: Refinement of the crystal structure of iodine at 100 °K. Acta Crystallogr. 23, 90–91 (1967)
El-Sheshtawy, H.S., Bassil, B.S., Assaf, K.I., Kortz, U., Nau, W.M.: Halogen bonding inside a molecular container. J. Am. Chem. Soc. 134, 19935–19941 (2012)
Hisaki, I., Suzuki, Y., Gomez, E., Ji, Q., Tohnai, N., Nakamura, T., Douhal, A.: Acid responsive hydrogen-bonded organic frameworks. J. Am. Chem. Soc. 141, 2111–2121 (2019)
Gomez, E., Suzuki, Y., Hisaki, I., Moreno, M., Douhal, A.: Spectroscopy and dynamics of a HOF and its molecular units: remarkable vapor acid sensing. J. Mater. Chem. C 7, 10818–10832 (2019)
Acknowledgements
The author thanks the organizing committee of Host-Guest and Supramolecular Chemistry Society, Japan for giving him the HGCS Japan Award of Excellence 2019 and the opportunity to write this review. This work was supported by Grant-in-Aid for Young Scientists (A) (No. JP24685026), for Scientific Research (C) and (B) (Nos. JP15K04591 and JP18H01966, respectively), and for Scientific Research on Innovative Areas “π-System Figuration” (No. JP15H00998) and “Coordination Asymmetry” (No. JP19H04557) from Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Japan Society for the Promotion of Science (JSPS). The author thanks to Prof. Abderrazzak Douhal at Universidad de Castilla-La Mancha for fluorescence spectroscopic experiments, Dr. Seiji Tsuzuki at the National Institute of Advanced Industrial Science and Technology (AIST) and Prof. Michio Katouda at Waseda University for theoretical calculation, Dr. Hiroyasu Sato at Rigaku Corporation for crystallographic analysis, Prof. Tomoki Ogoshi at Kyoto University for vapor sorption experiments, Prof. Takanori Fukushima at Tokyo Institute of Technology for his continuous encouragement during the π-System Figuration project and Dr. Seiki Baba, Dr. Nobuhiro Mizuno, Dr. Kunihisa Sugimoto, Dr. Shogo Kawaguchi, and Dr. Nobuhiro Yasuda at SPring-8 (JASRI) for synchrotron X-ray diffraction experiments. The author thanks Prof. Mikiji Miyata and Prof. Norimitsu Tohnai at Osaka University and Prof. Takayoshi Nakamura and Dr. Kiyonori Takahashi at Hokkaido University for helpful discussion, and all the group members. This is a paper selected for “HGCS Japan Award of Excellence 2019”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hisaki, I. Hydrogen-bonded porous frameworks constructed by rigid π-conjugated molecules with carboxy groups. J Incl Phenom Macrocycl Chem 96, 215–231 (2020). https://doi.org/10.1007/s10847-019-00972-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-019-00972-0