Abstract
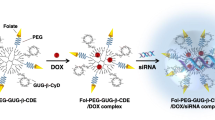
We previously reported the utility of 6-O-α-(4-O-α-d-glucuronyl)-d-glucosyl-β-cyclodextrin (GUG-β-CyD) conjugates with polyamidoamine dendrimer [GUG-β-CDE (generation 3; G3)] as siRNA carriers. In this study, to prepare GUG-β-CDE (G3) possessing a targeting ability to tumor cells overexpressing folate receptor-α (FR-α), we newly synthesized folate-polyethylene glycol (PEG)-appended GUG-β-CDEs (G3) [Fol-PEG-GUG-β-CDEs (G3)] having degrees of substitution of folate (DSF) of 3.9, 6.7 and 7.3, and evaluated their utility as tumor-selective siRNA carriers. Of various Fol-PEG-GUG-β-CDEs (G3), Fol-PEG-GUG-β-CDE (G3, DSF6.7) showed the highest siRNA transfection activity at a charge ratio of 50 (carrier/siRNA) in both 786-0-luc cells [FR-α (+)] and KB cells [FR-α (+)]. In addition, the cellular uptake of the complex was significantly decreased by an addition of folic acid in a concentration-dependent manner, suggesting its FR-α-mediated endocytosis pathway. Moreover, Fol-PEG-GUG-β-CDE (G3, DSF6.7)/siRNA complex induced a potent RNAi effect, comparable to Lipofectamine™ 2000/siRNA complex. Furthermore, Fol-PEG-GUG-β-CDE (G3, DSF6.7) complex with siRNA against Polo-like kinase 1 (siPLK1) showed a significant cytotoxic activity in KB cells. Thus, Fol-PEG-GUG-β-CDE (G3, DSF6.7) has the potential as the targeted siRNA delivery carrier for FR-α-overexpressing tumor cells.










Similar content being viewed by others
Abbreviations
- RNAi:
-
RNA interference
- siRNA:
-
Small interfering RNA
- FR:
-
Folate receptor
- FA:
-
Folic acid
- CyD:
-
Cyclodextrin
- GUG-β-CyD:
-
6-O-α-(4-O-α-d-Glucuronyl)-d-glucosyl-β-cyclodextrin
- PAMAM:
-
Polyamindoamine
- G:
-
Generation
- GUG-β-CDE:
-
GUG-β-CyD/dendrimer conjugate
- DS:
-
Degrees of substitution of GUG-β-CyD
- α-CDE:
-
α-CyD/dendrimer conjugate
- PEG:
-
Polyethylene glycol
- Fol-PEG-GUG-β-CDE:
-
Folate-PEG-appended GUG-β-CDE
- FBS:
-
Fetal bovine serum
- DSF:
-
Degrees of substitution of folate
- PLK1:
-
Polo-like kinase 1
- siPLK1:
-
siRNA against PLK1
References
Fire, A., Xu, S., Montgomery, M.K., Kostas, S.A., Driver, S.E., Mello, C.C.: Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998)
Ragelle, H., Riva, R., Vandermeulen, G., Naeye, B., Pourcelle, V., Le Duff, C.S., D’Haese, C., Nysten, B., Braeckmans, K., De Smedt, S.C., Jérôme, C., Préat, V.: Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J. Control. Release 176, 54–63 (2014)
Wu, S.Y., Lopez-Berestein, G., Calin, G.A., Sood, A.K.: Targeting the undruggable: advances and obstacles in current RNAi therapy. Sci. Transl. Med. 6, 240ps247–240ps247 (2014)
Caplen, N.J., Parrish, S., Imani, F., Fire, A., Morgan, R.A.: Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA 98, 9742–9747 (2001)
Lee, S.J., Kim, M.J., Kwon, I.C., Roberts, T.M.: Delivery strategies and potential targets for siRNA in major cancer types. Adv. Drug Deliv. Rev. 104, 2–15 (2016)
Al-Qadi, S., Grenha, A., Remuñán-López, C.: Chitosan and its derivatives as nanocarriers for siRNA delivery. J. Drug Deliv. Sci. Tech. 22, 29–42 (2012)
Conde, J., Ambrosone, A., Hernandez, Y., Tian, F., McCully, M., Berry, C.C., Baptista, P.V., Tortiglione, C., de la Fuente, J. M.: 15 years on siRNA delivery: beyond the state-of-the-art on inorganic nanoparticles for RNAi therapeutics. Nano Today 10, 421–450 (2015)
Chakraborty, C., Sharma, A.R., Sharma, G., Doss, C.G.P., Lee, S.-S.: Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol. Ther. Nucleic Acids 8, 132–143 (2017)
Bobbin, M.L., Rossi, J.J.: RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu. Rev. Pharmacol. Toxicol. 56, 103–122 (2016)
Kim, H.J., Kim, A., Miyata, K., Kataoka, K.: Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Deliv. Rev. 104, 61–77 (2016)
Quici, S., Casoni, A., Foschi, F., Armelao, L., Bottaro, G., Seraglia, R., Bolzati, C., Salvarese, N., Carpanese, D., Rosato, A.: Folic acid-conjugated europium complexes as luminescent probes for selective targeting of cancer cells. J. Med. Chem. 58, 2003–2014 (2015)
Mironava, T., Simon, M., Rafailovich, M.H., Rigas, B.: Platinum folate nanoparticles toxicity: cancer vs. normal cells. Toxicol. In Vitro 27, 882–889 (2013)
Ledermann, J.A., Canevari, S., Thigpen, T.: Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 26, 2034–2043 (2015)
Cheung, A., Bax, H.J., Josephs, D.H., Ilieva, K.M., Pellizzari, G., Opzoomer, J., Bloomfield, J., Fittall, M., Grigoriadis, A., Figini, M., Canevari, S., Spicer, J.F., Tutt, A.N., Karagiannis, S.N.: Targeting folate receptor a for cancer treatment. Oncotarget 7, 52553–52574 (2016)
Parker, N., Turk, M.J., Westrick, E., Lewis, J.D., Low, P.S., Leamon, C.P.: Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 338, 284–293 (2005)
Chen, C., Ke, J., Zhou, X.E., Yi, W., Brunzelle, J.S., Li, J., Yong, E.-L., Xu, H.E., Melcher, K.: Structural basis for molecular recognition of folic acid by folate receptors. Nature 500, 486–489 (2013)
Sun, L., Wu, Q., Peng, F., Liu, L., Gong, C.: Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloids Surf. B 135, 56–72 (2015)
Betzel, T., Müller, C., Groehn, V., Müller, A., Reber, J., Fischer, C.R., Krämer, S.D., Schibli, R., Ametamey, S.M.: Radiosynthesis and preclinical evaluation of 3′-aza-2′-[18F]fluorofolic acid: a novel PET radiotracer for folate receptor targeting. Bioconjug. Chem. 24, 205–214 (2013)
Toffoli, G., Cernigoi, C., Russo, A., Gallo, A., Bagnoli, M., Boiocchi, M.: Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer. 74, 193–198 (1998)
Chen, H., Ahn, R., Van den Bossche, J., Thompson, D.H., O’Halloran, T.V.: Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol. Cancer Ther. 8, 1955–1963 (2009)
Srinivasarao, M., Galliford, C.V., Low, P.S.: Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 14, 203–219 (2015)
Antony, A.C.: The biological chemistry of folate receptors. Blood 79, 2807–2820 (1992)
Abdelwahab, A.F., Ohyama, A., Higashi, T., Motoyama, K., Khaled, K.A., Sarhan, H.A., Hussein, A.K., Arima, H.: Preparation and evaluation of polyamidoamine dendrimer conjugate with glucuronylglucosyl-β-cyclodextrin (G3) as a novel carrier for siRNA. J. Drug Target. 22, 927–934 (2014)
Ishiguro, T., Fuse, T., Oka, M., Kurasawa, T., Nakamichi, M., Yasumura, Y., Tsuda, M., Yamaguchi, T., Nogami, I.: Synthesis of branched cyclomaltooligosaccharide carboxylic acids (cyclodextrin carboxylic acids) by microbial oxidation. Carbohydr. Res. 331, 423–430 (2001)
Ohyama, A., Higashi, T., Motoyama, K., Arima, H.: In vitro and in vivo tumor-targeting siRNA delivery using folate-PEG-appended dendrimer (G4)/α-cyclodextrin conjugates. Bioconjug. Chem. 27, 521–532 (2016)
Arima, H., Chihara, Y., Arizono, M., Yamashita, S., Wada, K., Hirayama, F., Uekama, K.: Enhancement of gene transfer activity mediated by mannosylated dendrimer/α-cyclodextrin conjugate (generation 3, G3). J. Control. Release 116, 64–74 (2006)
Ishiyama, M., Tominaga, H., Shiga, M., Sasamoto, K., Ohkura, Y., Ueno, K.: A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 19, 1518–1520 (1996)
Reddy, J.A., Low, P.S.: Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit. Rev. Ther. Drug Carrier Syst. 15, 587–627 (1998)
Ohyama, A., Higashi, T., Motoyama, K., Arima, H.: Ternary complexes of folate-PEG-appended dendrimer (G4)/α-cyclodextrin conjugate, siRNA and low-molecular-weight polysaccharide sacran as a novel tumor-selective siRNA delivery system. Int. J. Biol. Macromol. 99, 21–28 (2017)
Shen, X.-C., Zhou, J., Liu, X., Wu, J., Qu, F., Zhang, Z.-L., Pang, D.-W., Quelever, G., Zhang, C.-C., Peng, L.: Importance of size-to-charge ratio in construction of stable and uniform nanoscale RNA/dendrimer complexes. Org. Biomol. Chem. 5, 3674–3681 (2007)
Lee, D.J., Kessel, E., Lehto, T., Liu, X., Yoshinaga, N., Padari, K., Chen, Y.C., Kempter, S., Uchida, S., Rädler, J.O., Pooga, M., Sheu, M.T., Kataoka, K., Wagner, E.: Systemic delivery of folate-PEG siRNA lipopolyplexes with enhanced intracellular stability for in vivo gene silencing in leukemia. Bioconjug. Chem. 28, 2393–2409 (2017)
Anno, T., Higashi, T., Motoyama, K., Hirayama, F., Uekama, K., Arima, H.: Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a DNA carrier in vitro and in vivo. J. Drug Target. 20, 272–280 (2012)
Anno, T., Higashi, T., Motoyama, K., Hirayama, F., Uekama, K., Arima, H.: Possible enhancing mechanisms for gene transfer activity of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate. Int. J. Pharm. 426, 239–247 (2012)
Anno, T., Higashi, T., Hayashi, Y., Motoyama, K., Jono, H., Ando, Y., Arima, H.: Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a siRNA carrier for the treatment of familial amyloidotic polyneuropathy. J. Drug Target. 22, 883–890 (2014)
Takai, N., Hamanaka, R., Yoshimatsu, J., Miyakawa, I.: Polo-like kinases (Plks) and cancer. Oncogene 24, 287–291 (2005)
Lacaze, P., Raza, S., Sing, G., Page, D., Forster, T., Storm, P., Craigon, M., Awad, T., Ghazal, P., Freeman, T.C.: Combined genome-wide expression profiling and targeted RNA interference in primary mouse macrophages reveals perturbation of transcriptional networks associated with interferon signalling. BMC Genomics 10, 372 (2009)
Jensen, K., Anderson, J.A., Glass, E.J.: Comparison of small interfering RNA (siRNA) delivery into bovine monocyte-derived macrophages by transfection and electroporation. Vet. Immunol. Immunopathol. 158, 224–232 (2014)
Jevprasesphant, R., Penny, J., Jalal, R., Attwood, D., McKeown, N.B., D’Emanuele, A.: The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm. 252, 263–266 (2003)
Maruyama, K., Iwasaki, F., Takizawa, T., Yanagie, H., Niidome, T., Yamada, E., Ito, T., Koyama, Y.: Novel receptor-mediated gene delivery system comprising plasmid/protamine/sugar-containing polyanion ternary complex. Biomaterials 25, 3267–3273 (2004)
Acknowledgements
The authors thank Ensuiko Sugar Refining for providing GUG-CyD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohammed, A.F.A., Higashi, T., Motoyama, K. et al. Targeted siRNA delivery to tumor cells by folate-PEG-appended dendrimer/glucuronylglucosyl-β-cyclodextrin conjugate. J Incl Phenom Macrocycl Chem 93, 41–52 (2019). https://doi.org/10.1007/s10847-018-0834-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0834-9