Abstract
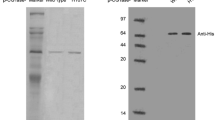
Cyclodextrin glucanotransferase (CGTase, EC 2.4.1.19) is an enzyme that degrades starch and starch related glucans into cyclodextrins (CDs) by intramolecular transglycosylation reaction. The biochemical activity of recombinant CGTase from Anaerobranca gottschalkii for the yield and product specificity of cyclodextrins was investigated in the presence of organic solvents. Compared with the control of starch bioconversion, addition of various organic solvents generally increased the total CD and product specificity by affecting product inhibition and/or intermolecular transglycosylation reaction. The highest conversion (45 %) of starch to CDs was obtained in the presence of ethanol, while the simultaneous addition of two organic solvents, decanol-ethanol, comparatively showed a reduced total yield of 39 %. Despite this, the highest product ratio of 91 % α-CD, and 64 % β-CD was obtained in the presence of decanol and cyclohexane respectively. The effect of organic solvents on the yield and specificity of CD was attributed mainly to their effect on product inhibition and transglycosylation reaction. Although the use of two organic solvents showed almost a significant increase in total yield of CDs, it resulted in a comparatively lower specific product yield compared to their respective individual effect. Generally, normal enzyme activity was favoured at higher temperature of 65 °C, but the addition of organic solvents, in most cases, was found to decrease the bioconversion. Thus, the preferred optimal condition was reduced to 40 °C, where the maximal conversion of starch to CDs in general and α-CD in particular was achieved.
Similar content being viewed by others
References
Li, Z., et al.: Extracellular expression and biochemical characterization of α-cyclodextrin glycosyltransferase from Paenibacillus macerans. Carbohydr. Res. 345, 886–892 (2010)
Kuo, C–.C., et al.: Production of cyclodextrin glucanotransferase from an alkalophilic Bacillus sp. by pH-stat fed-batch fermentation. Biotechnol. Lett. 31, 1723–1727 (2009)
Valle, E.M.M.D.: Cyclodextrins and their uses: a review. Process. Biochem. 39, 1033–1046 (2004)
Moriwaki, C., et al.: A novel cyclodextrin glycosyltransferase from Bacillus sphaericus strain 41: production, characterization and catalytic properties. Biochem. Eng. J. 48, 124–131 (2009)
Charoensakdi, R., et al.: A recombinant cyclodextrin glycosyltransferase cloned from Paenibacillus sp. strain RB01 showed improved catalytic activity in coupling reaction between cyclodextrins and disaccharides. J. Incl. Phenom. Macrocycl. Chem. 57, 53–59 (2007)
Tardioli, P.W., Zanin, G.M., Moraes, FFd: Characterization of thermoanaerobacter cyclomaltodextrin glucanotransferase immobilized on glyoxyl-agarose. Enzyme. Microb. Technol. 39, 1270–1278 (2006)
Blackwood, A.D., Bucke, C.: Addition of polar organic solvents can improve the product selectivity of cyclodextrin glycosyltransferase. Solvent effects on CGTase. Enzyme. Microb. Technol. 27, 704–708 (2000)
Biwer, A., Antranikian, G., Heinzle, E.: Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 59, 609–617 (2002)
Doukyu, N., Ogino, H.: Organic solvent-tolerant enzymes. Biochem. Eng. J. 48, 270–282 (2010)
Kaulpiboon, J., Hansakul, P.: Comparative studies on the synthesis of cyclodextrin from two bacterial CGTases in the presence of organic solvents. Thammasat. Int. J. Sc. Tech. 12(2), 10–17 (2007)
Qi, Q., Zimmermann, W.: Cyclodextrin glucanotransferase: from gene to applications. Appl. Microbiol. Biotechnol. 66, 475–485 (2005)
Thiemann, V., et al.: Characterisation of a thermoalkali-stable cyclodextrin glycosyltransferase from the anaerobic thermoalkaliphilic bacterium Anaerobranca gottschalkii. Arch. Microbiol. 182, 226–235 (2004)
Ding, R., et al.: Enhanced secretion of recombinant α-cyclodextrin glucosyltransferase from E. coli by medium additives. Process. Biochem. 45, 880–886 (2010)
Rahman, K., et al.: Molecular cloning of a cyclodextrin glucanotransferase gene from alkalophilic Bacillus sp. TS1-1 and characterization of the recombinant enzyme. Enzyme. Microb. Technol. 39, 74–84 (2006)
Cao, X., et al.: A novel cyclodextrin glycosyltransferase from an alkalophilic Bacillus species: purification and characterization. Food. Res. Int. 38, 309–314 (2005)
Hannig, G., Makrides, S.C.: Strategies for optimizing heterologous protein expression in Escherichia coli. Trends. Biotechnol. 16(2), 54–60 (1998)
Kelly, R.M., Dijkhuizen, L., Leemhuis, H.: The evolution of cyclodextrin glucanotransferase product specificity. Appl. Microbiol. Biotechnol. 84, 119–133 (2009)
Astray, G., et al.: A review on the use of cyclodextrins in foods. Food. Hydrocolloid. 23, 1631–1640 (2009)
Bruce, L.Z., Henrik, K.N., Robert, L.S.: Thermostable enzymes for industrial applications. J. Ind. Microbiol. 8, 71–82 (1991)
Giordano, F., Novak, C., Moyano, J.R.: Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta. 380, 123–151 (2001)
Goh, K.M., et al.: The effects of reaction conditions on the production of γ-cyclodextrin from tapioca starch by using a novel recombinant engineered CGTase. J. Mol. Catal. B Enzym. 49, 118–126 (2007)
Hamilton, L.M., Kelly, C.T., Fogarty, W.M.: Review: cyclodextrins and their interaction with amylolytic enzymes. Enzyme. Microb. Technol. 26, 561–567 (2000)
Hedges, A.R.: Industrial applications of cyclodextrins. Chem. Rev. 98, 2035–2044 (1998)
Jana, S., Deb, J.K.: Strategies for efficient production of heterologous proteins in Escherichia coli. Appl. Microbiol. Biotechnol. 67, 289–298 (2005)
Kim, M.H., Sohn, C.B., Oh, T.K.: Cloning and sequencing of a cyclodextrin glycosyltransferase gene from Brevibacillus brevis CD162 and its expression in Escherichia coli. FEMS. Microbiol. Lett. 164, 411–418 (1998)
Leemhuis, H., Kelly, R.M., Dijkhuizen, L.: Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications. Appl. Microbiol. Biotechnol. 85, 823–835 (2010)
Li, Z.-F., et al.: Calcium Leads to further increase in glycine-enhanced extracellular secretion of recombinant α-cyclodextrin glycosyltransferase in Escherichia coli. J. Agric. Food. Chem. 57, 6231–6237 (2009)
Loftsson, T., Duchêne, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329, 1–11 (2007)
Ong, R.M., et al.: Cloning, extracellular expression and characterizationof a predominant α-CGTase from Bacillus sp. G1 in E. coli. J. Ind. Microbiol. Biotechnol. 35, 1705–1714 (2008)
Pishtiyski, I., Zhekova, B.: Effect of different substrates and their preliminary treatment on cyclodextrin production. World. J. Microbiol. Biotechnol. 22, 109–114 (2006)
Szejtli, J.z.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Tonkova, A.: Bacterial cyclodextrin glucanotransferase. Enzyme. Microb. Technol. 22, 678–686 (1998)
Veen, BAvd, et al.: The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur. J. Biochem. 267, 658–665 (2000)
Veen, BAvd, et al.: The role of arginine 47 in the cyclization and coupling reactions of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 Implications for product inhibition and product specificity. Eur. J. Biochem. 267, 3432–3441 (2000)
Veen, BAvd, et al.: Engineering of cyclodextrin glycosyltransferase reaction and product specificity. Biochim. Biophys. Acta. 1543(2), 336–360 (2000)
Villalonga, R., Cao, R., Fragoso, A.: Supramolecular chemistry of cyclodextrins in enzyme technology. Chem. Rev. 107, 3088–3116 (2007)
Zhekova, B., et al.: Approaches for yield increase of β -cyclodextrin formed by cyclodextrin glucanotransferase from Bacillus megaterium. World. J. Microbiol. Biotechnol. 25, 1043–1049 (2009)
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (30970057) and (31100048), the Key Technologies R & D Program of Jiangsu Province, China (BE2011711), the Key Program of National Natural Science Foundation of China (20836003), and the 111 Project (No. 111-2-06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tesfai, B.T., Wu, D., Chen, S. et al. Effect of organic solvents on the yield and specificity of cyclodextrins by recombinant cyclodextrin glucanotransferase (CGTase) from Anaerobranca gottschalkii . J Incl Phenom Macrocycl Chem 77, 147–153 (2013). https://doi.org/10.1007/s10847-012-0225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0225-6