Abstract
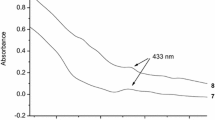
In the presence of small amount of 1-iodo butane (IBu) (0.1 % (v/v)), Naproxen (Nap) displays strong room temperature phosphorescence (RTP) in β-cyclodextrin (β-CD) solution without deoxygenation because of the formation of ternary complex of β-CD, Nap, and IBu. The results indicate that β-CD shows good enantiodiscrimination for (R)-Nap and (S)-Nap. The RTP intensity of (R)-Nap is larger than that of (S)-Nap, the difference being 29.2 %. Both (R)-Nap and (S)-Nap exhibit single exponential phosphorescence decay with different lifetimes of 2.535 ± 0.056 and 1.798 ± 0.076 ms for (R)-Nap and for (S)-Nap, respectively. The corresponding association constants evaluated for (R)-Nap/β-CD/IBu and (S)-Nap/β-CD/IBu ternary complexes are (8.02 ± 0.15) × 103 and (2.50 ± 0.06) × 103 L mol−1, respectively. Thus, the observation of RTP differences between (R)-Nap and (S)-Nap can be attributed to their different ability to form complexes with chiral β-CD.











Similar content being viewed by others
References
Cirri, M., Maestrelli, F., Corti, G., Furlanetto, S., Mura, P.: Simultaneous effect of cyclodextrin complexation, pH, and hydrophilic polymers on naproxen solubilization. J. Pharm. Biomed. Anal. 42, 126–131 (2006)
Cheng, X.L., Zhao, L.X., Liu, M.L., Lin, J.M.: In vitro monitoring of nanogram levels of naproxen in human urine using flow injection chemiluminescence. Anal. Chim. Acta 558, 296–301 (2006)
Loll, P.J., Picot, D., Ekabo, O., Garavito, R.M.: Synthesis and use of iodinated nonsteroidal anti-inflammatory drug analogues as crystallographic probes of the prostaglandin H2 synthase cyclooxygenase active site. Biochemistry 35, 7330–7340 (1996)
Harrison, I.T., Lewis, B., Nelson, P., Rooks, W., Roszkowski, A., Tomolonis, A., Fried, J.H.: Nonsteroidal antiinflammatory agents. I. 6-Substituted 2-naphthyl acetic acids. J. Med. Chem. 13, 203–205 (1970)
Cui, Y.M., Wei, D.Z., Yu, J.T.: Lipase-catalyzed esterification in organic solvent to resolve racemic naproxen. Biotechnol. Lett. 19, 865–868 (1997)
González, S., Pelaéz, R., Sanz, F., Jiménez, M.B., Morán, J.R., Caballero, M.C.: Macrocyclic chiral receptors toward enantioselective recognition of naproxen. Org. Lett. 8, 4679–4682 (2006)
Li, S., Purdy, W.C.: Cyclodextrins and their applications in analytical chemistry. Chem. Rev. 92, 1457–1470 (1992)
Liu, Y., Li, L., Zhang, H.Y., Fan, Z., Guan, X.D.: Selective binding of chiral molecules of cinchona alkaloid by β- and γ-cyclodextrins and organoselenium-bridged bis(β-cyclodextrin)s. Bioorg. Chem. 31, 11–23 (2003)
Bakirci, H., Nau, W.M.: Chiral discrimination in the complexation of heptakis-(2, 6-di-O -methyl)-β-cyclodextrin with 2, 3-diazabicyclo[2.2.2]oct-2-ene derivatives. J. Photochem. Photobio. A 173, 340–348 (2005)
Fan, Y.X., Yang, Y., Shuang, S.M., Dong, C.: Molecular recognition of α-cyclodextrin (CD) to chiral amino acids based on methyl orange as a molecular probe. Spectrochim. Acta 61, 953–959 (2005)
Kumar, V.P., Suryanarayana, I., Nageswar, Y.V.D., Rao, K.R.: Chiral discrimination of tolterodine tartrate by modified cyclodextrins. J. Carbohydr. Chem. 27, 223–230 (2008)
Sun, P., MacDonnell, F.M., Armstrong, D.W.: Enantioselective host–guest complexation of Ru(II) tris diimine complexes using neutral and anionic derivatized cyclodextrins. Inorg. Chim. Acta 362, 3073–3078 (2009)
García-Ruiz, C., Hu, X.S., Ariese, F., Gooijer, C.: Enantioselective room temperature phosphorescence detection of non-phosphorescent analytes based on interaction with β-cyclodextrin/1-bromo naphthalene complexes. Talanta 66, 634–640 (2005)
García-Ruiz, C., Siderius, M., Ariese, F., Gooijer, C.: Quenched phosphorescence as a detection method in capillary electrophoretic chiral separations. Monitoring the stereoselective biodegradation of camphorquinone by yeast. Anal. Chem. 76, 399–403 (2004)
García-Ruiz, C., Scholtes, M.J., Ariese, F., Gooijer, C.: Enantioselective detection of chiral phosphorescent analytes in cyclodextrin complexes. Talanta 66, 641–645 (2005)
Lammers, I., Buijs, J., van der Zwan, G., Ariese, F., Gooijer, C.: Phosphorescence for sensitive enantioselective detection in chiral capillary electrophoresis. Anal. Chem. 81, 6226–6233 (2009)
Zhang, X.H., Wang, Y., Jin, W.J.: Chiral discrimination of quinine and quinidine based on notable room temperature phosphorescence lifetime differences with γ-cyclodextrin as chiral selector. Talanta 73, 938–942 (2007)
Zhang, X.H., Wang, Y., Jin, W.J.: Enantiomeric discrimination of 1,1′-binaphthol by room temperature phosphorimetry using γ-cyclodextrin as chiral selector. Anal. Chim. Acta 622, 157–162 (2008)
Wang, Y., Feng, T.T., Chao, J.B., Qin, L.P., Zhang, Z., Jin, W.J.: Phosphorescence properties and chiral discrimination of camphorquinone enantiomers in the presence of α-cyclodextrin and 1,2-dibromoethane. J. Photochem. Photobio. A Chem. 212, 49–55 (2010)
Wang, J., Warner, I.M.: Studies of the naproxen: β-Cyclodextrin inclusion complex. Microchem. J. 48, 229–239 (1993)
Sadlej-Sosnowska, N., Kozerski, L., Bednarek, E., Sitkowski, J.: Fluorometric and NMR studies of the naproxen–cyclodextrin inclusion complexes in aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 37, 383–394 (2000)
Bettinetti, G., Melani, E., Mura, E., Monnanni, R., Giordano, E.: Carbon-13 nuclear magnetic resonance study of naproxen interaction with cyclodextrins in solution. J. Pharm. Sci. 80, 1162–1170 (1991)
Junco, S., Casimiro, T., Ribeiro, N., Ponte, M.N.D., Marques, H.C.: A comparative study of naproxen-beta cyclodextrin complexes prepared by conventional methods and using supercritical carbon dioxide. J. Incl. Phenom. Macrocycl. Chem. 44, 117–121 (2002)
Rapado Martínez, I., Villanueva Camañas, R.M., García-Alvarez-Coque, M.C.: Micelle-stabilized room-temperature phosphorimetric procedure for the determination of naproxen and propranolol in pharmaceutical preparations. Analyst 119, 1093–1097 (1994)
Carretero, A.S., Cruces-Blanco, C., Ramirez, M.I., Diaz, G.B.C., Fernández-Gutiérrez, A.: Simple and rapid determination of the drug naproxen in pharmaceutical preparations by heavy atom-induced room temperature phosphorescence. Talanta 50, 401–407 (1999)
Pérez-Ruiz, T., Martínez Lozano, C., Tomás, V., Carpena, J.: Selective determination of naproxen in the presence of nonsteroidal anti-inflammatory drugs in serum and urine samples using room temperature liquid phosphorimetry. J. Pharm. Biomed. Anal. 17, 719–724 (1998)
Bussey III, R.O., Schuh, M.D.: Quantitation of naproxen by quenching of phosphorescence from a ternary complex of 2-bromo-6-methoxynaphthalene and α-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 57, 163–167 (2007)
Segura Carretero, A., Cruces-Blanco, C., Cañabate-Díaz, B., Fernández-Sánchez, J.F., Fernández-Gutiérrez, A.: Heavy-atom induced room-temperature phosphorescence: a straightforward methodology for the determination of organic compounds in solution. Anal. Chim. Acta 417, 19–30 (2000)
Arancibia, J.A., Escandar, G.M.: Determination of naproxen in pharmaceutical preparations by room-temperature phosphorescence. A comparative study of several organized media. Analyst 126, 917–922 (2001)
Segura Carretero, A., Cruces-Blanco, C., Ramírez Garcıía, M.I., Diaz, B.C., Fernández-Gutiérrez, A.: Simple and rapid determination of the drug naproxen in pharmaceutical preparations by heavy atom-induced room temperature phosphorescence. Talanta 50, 401–407 (1999)
Jiménez, M.C., Pischel, U., Miranda, M.A.: Photoinduced processes in naproxen-based chiral dyads. J. Photochem. Photobio. C 8, 128–142 (2007)
Valero, M., López-Cornejo, M.P., Costa, S.M.B.: Effect of the structure and concentration of cyclodextrins in the quenching process of naproxen. J. Photochem. Photobio. A 188, 5–11 (2007)
Fernández-Sánchez, J.F., Segura-Carretero, A., Cruces-Blanco, C., Fernández-Gutiérrez, A.: Room-temperature luminescence optosensings based on immobilized active principles actives. Application to nafronyl and naproxen determination in pharmaceutical preparations and biological Fluids. Anal. Chim. Acta 462, 217–224 (2002)
Banerjee, T., Singh, S.K., Kishore, N.: Binding of naproxen and amitriptyline to bovine serum albumin: biophysical aspects. J. Phys. Chem. B 110, 24147–24156 (2006)
Wen, X.H., Tan, F., Jing, Z.J., Liu, Z.Y.: Preparation and study the 1:2 inclusion complex of carvedilol with β-cyclodextrin. J. Pharma. Biomed. Anal. 34, 517–523 (2004)
Acknowledgments
This work was financially supported by the Natural Science Foundation of Shanxi Province (No. 2008011015-1), the Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry and Research Project Supported by Shanxi Scholarship Council of China (2011-008).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Feng, T.T., Shi, L.L. et al. Enantioselective phosphorescence behavior of naproxen in β-cyclodextrin supramolecular complex. J Incl Phenom Macrocycl Chem 76, 151–158 (2013). https://doi.org/10.1007/s10847-012-0184-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0184-y