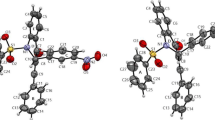
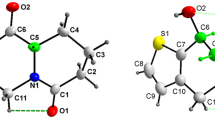
Abstract
A series ten novel analogs based on a novel template pyrrolo-quinazolino-quinolines, containing Luotonin-A (Luot-A) and 14-aza-camptothecin (14-aza-CPT) molecular core as well as their inclusion complexes in the native α- (α-CD), β- (β-CD) and γ- (β-CD) cyclodextrins were obtained. The physical properties of the alkaloids and corresponding molecular complexes with cyclodextrins are elucidated experimentally by the electronic absorption and CD-spectroscopy, electrospray ionization and matrix-assisted laser desorption/ionization mass spectrometry and nuclear magnetic resonance method. The experimental data are supported by the theoretical quantum chemical calculations of the molecular and electronic structures as well as physical properties in condense phase.





Similar content being viewed by others
References
Ma, Z., Hano, Y., Nomura, T., Chen, Y.: Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles 46, 541 (1997)
Ma, Z., Hano, Y., Nomura, T., Chen, Y.: Two new quinazoline-quinoline alkaloids from Peganum nigellastrum. Heterocycles 51, 1883 (1999)
Ali Tasneem, M., Rajanna, K., SaiPrakash, P.: An efficient and facile synthesis of 2-chloro-3-formyl quinolines from acetanilides in micellar media by Vils-meier-HaackCyclisation. Synlett 32, 251 (2001)
Amin, A., Mehta, D., Samarth, S.: Vasicineand related compounds. Prog. Drug. Res. 14, 218 (1970)
Johne, S.: Search for pharmaceutically interesting quinazoline derivatives: efforts and results (1969–1980). Prog. Drug. Res. 26, 259 (1982)
Govindachari, T., Ravindranath, K., Viswanathan, N.: Mappicine, a minor alkaloid from Mappia foetida miers. J. Chem. Soc. Perkin Trans. 1, 1215 (1974)
Hertzberg, R., Caranfa, M., Holden, K., Jakas, D., Gallagher, G., Mattern, M.R., Mong, S., Bartus, J., Johnson, R., Kingsbury, W.J.: Modification of the hydroxylactone ring of camptothecin: inhibition of mammalian topoisomerase I and biological activity. J. Med. Chem. 32, 715 (1989)
Adamovics, J., Hutchinson, C.J.: Prodrug analogs of the antitumor alkaloid camptothecin. J. Med. Chem. 22, 310 (1979)
Sawada, S., Yaegashi, T., Furuta, T., Yokokura, T., Miyasaka, T.: Chemical modification of an antitumor alkaloid, 20(S)-camptothecin: E-lactone ring-modified water-soluble derivatives of 7-ethylcamptothecin. Chem. Pharm. Bull. 41, 310 (1993)
Nicholas, A., Wani, M., Manikumar, G., Wall, M., Kohn, K., Pommier, Y.: Plant antitumor agents. 29. Synthesis and biological activity of ring D and ring E modified analogs of camptothecin. J. Med. Chem. 33, 972 (1990)
Ejima, A., Terasawa, H., Sugimori, M., Ohsuki, S., Matsumoto, K., Kawato, Y., Yasuoka, M., Tagawa, H.: Antitumor agents. V. Synthesis and antileukemic activity of E-ring-modified (RS)-camptothecin analogues. Chem. Pharm. Bull. 41, 683 (1992)
Cagir, A., Jones, S., Gao, R., Eisenhauer, B., Hecht, S.J.: Communication luotonin A. a naturally occurring human dna topoisomerase I poison. J. Am. Chem. Soc. 125, 13628 (2003)
Dallavalle, S., Merlini, L.: A new synthesis of the cytotoxic alkaloid luotonine A. Tetrahedron Lett. 43, 1835 (2002)
Yadav, J., Reddy, B.: Microwave-assisted rapid synthesis of the cytotoxic alkaloid luotonin A. Tetrahedron Lett. 43, 1905 (2002)
Osborne, D., Stevenson, P.: Concise formal synthesis of luotonin A. Tetrahedron Lett. 43, 5469 (2002)
Lee, E., Park, J., Jahng, Y.: A facile synthesis of simple alkaloids—synthesis of 2,3-polymethylene-4(3H)-quinazolinones and related alkaloids. Tetrahedron Lett. 44, 1883 (2003)
Chavan, S., Sivappa, R.: A short and efficient general synthesis of luotonin A, B and E. Tetrahedron 60, 9931 (2004)
Liang, J., Cha, H., Jahng, Y.: Recent advances in the studies on luotonins. Molecules 16, 4861 (2011)
Cortesi, R., Esposito, E., Maietti, A., Menegatti, E., Nastruzzi, C.: Formulation study for the antitumor drug camptothecin: liposomes, micellar solutions and a microemulsion. Int. J. Pharm. 159, 95 (1997)
Burke, T., Staubus, A., Misra, A.: Liposomal stabilization of camptothecin’s lactone ring. J. Am. Chem. Soc. 114, 8318 (1992)
Daoud, S., Fetouh, M., Giovanella, B.: Antitumor effect of liposome-incorporated camptothecin in human malignant xenografts. Anti Cancer Drugs 6, 83 (1995)
Kang, J., Kumar, V., Yang, D., Chowdhury, P., Hohl, R.: Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur. J. Pharm. Sci. 15, 163 (2002)
Steffen, A., Thiele, B., Tietze, S., Strassnig, C., Kämper, A., Lengauer, T., Wenz, G., Apostolakis, J.: Apostolakis mproved cyclodextrin-based receptors for camptothecin by inverse virtual screening. Chem. Eur. J. 13, 6801 (2007)
Foulon, C., Tedou, T., Queruau, T., Vaccher, C., Bonte, F., Goossens, J.: Assessment of the complexation degree of camptothecin derivatives and cyclodextrins using spectroscopic and separative methodologies. Tetrahedron Asymm. 20, 2482 (2009)
Xiang, T., Anderson, B.: Stable supersaturated aqueous solutions of silatecan 7-t-butyldimethylsilyl-10-hydroxycamptothecin via chemical conversion in the presence of a chemically modified beta-cyclodextrin. Pharm. Res. 19, 1215 (2002)
Tong, R., Cheng, J.: Controlled synthesis of camptothecin–polylactide conjugates and nanoconjugates. Bioconj. Chem. 21, 111 (2010)
Mussardo, P., Corda, E., González-Ruiz, V., Rajesh, J., Girotti, S., Martín, M., Olives, A.: Study of non-covalent interactions of luotonin A derivatives and the DNA minor groove as a first step in the study of their analytical potential as DNA probes. Anal. Bioanal. Chem 400, 321 (2011)
Szente, L., Szetjili, J.: Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 36, 17–28 (1999)
Szente, L., Vikmon, M., Szeman, J., Otta, K.S.T.P.: Methods to enhance the complexation efficiency of cyclodextrins. Pharm. Sci. 9, 243 (1999)
Thompson, D.: Cyclodextrins enabling excipients: their present and future use in pharmaceuticals. Crit. Rev. Ther. Drug Carr. Syst. 14, 1 (1997)
Awouafack, M., Spiteller, P., Lamshöft, M., Kusari, S., Ivanova, B., Tane, P., Spiteller, M.J.: Antimicrobial isopropenyl-dihydrofuranoisoflavones from Crotalaria lachnophora. Nat. Prod. 74, 272 (2011)
Awouafack, M., Kusari, S., Lamshöft, M., Ngamga, D., Tane, P., Spiteller, M.: Semi-synthesis of dihydrochalcone derivatives and their in vitro antimicrobial activities. Planta Medica 76, 640 (2010)
Kusari, S., Zühlke, S., Spiteller, M.: An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J. Nat. Prod. 72, 2 (2009)
Sawada, S., Okajama, S., Aijama, R., Nokata, K., Furuta, T., Yokokura, T., Sugino, E., Yamaguchi, K., Miyasaka, Y.: Synthesis and antitumor activity of 20(S) substituted camptothecin. Chem. Pharm. Bull. 39, 1446 (1991)
Ivanova, B., Spiteller, M.: Conformation, optical properties, and absolute configuration of 2′,3′-isopropylideneadenosines: theoretical versus experimental study. J. Mol. Struct. 1004, 303 (2011)
Ivanova, B., Spiteller, M.: Structure and properties of camptothecin derivatives, their protonated forms, and model interaction with the topoisomerase I-DNA complex. Biopolymers 97, 134 (2012)
Aiyama, R., Nagai, H., Sawada, N., Yokokura, T., Itokawa, H., Nakanishi, M.: Determination of self-association of irinotecan hydrochloride (CPT-11) in aqueous solution. Chem. Pharm. Bull. 40, 2810–2813 (1992)
Ivanova, B., Spiteller, M.J.: Experimental and theoretical spectroscopic and structural study of A-ring substituted camptothecins. Mol. Struct. 1012, 189 (2012)
Ivanova, B., Spiteller, M.: Physical properties and molecular conformations of indole alkaloids and model protein interactions–theoretical versus experimental study. Nat. Prod. Commun. 7, 1 (2012)
Kumara, P., Zuehlke, S., Priti, V., Ramesha, B., Shweta, S., Ravikanth, B., Vasudeva, R., Santhoshkumar, Spiteller, M., Shaanker, R.: Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook.f, produces rohitukine, a chromane alkaloid possessing anti-cancer activity. Antonie van Leewenhoek Int. J. General Mol. Microbiol. 101, 323 (2012)
Mason, J., Bergman, J.: Synthesis and biological activities of natural and non-natural quinazolines. Org. Biomol. Chem. 5, 2486 (2007)
Yoon, K., Krull, K., Morton, C., Bornmann, W., Lee, R., Potter, P., Danks, M.: Activation of a camptothecin prodrug by specific carboxylesterases as predicted by quantitative structure-activity relationship and molecular docking studies. Mol. Cancer. Ther. 2, 1171 (2003)
Curran, D., Ko, S., Josien, H.: Cascade radical reactions of isonitriles: a second-generation synthesis of (20S)-camptothecin, topotecan, irinotecan, and GI-147211C. Angew. Chem. Int. Ed. 34, 2683 (1995)
Sawada, S., Yokokura, T., MIiyasaka, T.: Synthesis of CPT-11. Ann. New York Acad. Sci. 803, 13 (1996)
Sawada, H., Watanabe, T., Yokokura, T.: Structure-activity relationships of N-(3,5-dimethoxy-4-n-octyloxycinnamoyl)-N’-(3,4-dimethylphenyl)piperazine and analogues as inhibitors of acyl-CoA: cholesterol O-acyltransferase. Chem. Pharm. Bull. 49, 830 (2001)
Sawada, D., Tsukuda, Y., Saito, H., Takagi, K., Horie, K., Ochiai, E., Takenouchi, K., Kittaka, K.: Synthesis and biological evaluation of 4-substituted vitamin d and 14-epi-previtamin d analogs. Chem. Pharm. Bull. 57, 1431 (2009)
Sawada, K., Okada, S., Kuroda, A., Watanabe, S., Sawada, Y., Tanaka, H.: 4-(Benzoylindolizinyl)butyric acids; novel nonsteroidal inhibitors of steroid 5alpha-reductase. III. Chem. Pharm. Bull. 49, 799 (2001)
Rahman, A., Kim, D., Liang, J., Lee, E., Na, Y., Jun, K., Kwon, Y., Jahng, Y.: Synthesis and biological properties of luotonin a derivatives. Bull. Korean Chem. Soc. 29, 1988 (2008)
Samori, C., Guerrini, A., Varchi, G., Zunino, F., Beretta, G., Femoni, C., Bombardelli, E., Fontana, G., Battaglia, A.: Thiocamptothecin. J. Med. Chem. 51, 3040 (2008)
Cinelli, M., Cordero, B., Dexheimer, T., Pommier, Y., Cushman, M.: Synthesis and biological evaluation of 14-(aminoalkyl-aminomethyl)aromathecins as topoisomerase I inhibitors: investigating the hypothesis of shared structure-activity relationships. Bioorg. Med. Chem. 15, 7145 (2009)
Rahier, N., Cheng, K., Gao, R., Eisenhauer, B., Hecht, S.: Synthesis of 14-azacamptothecin, a water-soluble topoisomerase i poison. Org. Lett. 7, 835 (2005)
Elban, M., Sun, V., Eisenhauer, B., Gao, R., Hecht, S.: Synthesis and biological evaluation of 10,11-methylenedioxy-14-azacamptothecin. Org. Lett. 8, 3513 (2006)
Dodds, H., Haaz, M., Riou, J., Robert, J., Rivori, L.: Identification of a new metabolite of CPT-11 (irinotecan): pharmacological properties and activation to SN-38. J. Pharmacol. Exp. Therap. 286, 578 (1998)
Rivory, L., Haaz, M., Canal, P., Lokiec, F., Armand, J., Robert, J.: Pharmacokinetic interrelationships of irinotecan (CPT-11) and its three major plasma metabolites in patients enrolled in phase I/II trials. Clin. Cancer. Res. 3, 1261 (1997)
Santos, A., Zanetta, S., Cresteil, T., Deroussent, A., Pein, P., Raymond, E., Vernillet, L., Risse, M., Boige, V., Gouyette, A., Vassal, V.: Metabolism of irinotecan (CPT-11) by CYP3A4 and CYP3A5 in humans. Clin. Cancer Res. 6, 2012 (2000)
Cole, R. (ed.): Electrospray and MALDI Mass Spectrometry, 2nd edn. Wiley, New York (2010)
Srimany, A., Ifa, D., Naik, H., Bhat, V., Cooks, R., Pradeep, T.: Direct analysis of camptothecin from nothapodytes nimmoniana by desorption electrospray ionization mass spectrometry (DESI-MS). Analyst 136, 3066 (2011)
Frisch M, et al.: Gaussian 09w, Gaussian Inc., Pittsburgh (2009)
Dalton 2.0 Program package
Zhao, Y., Truhlar, D.: Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157 (2008)
Schultz, N., Zhao, Y., Truhlar, D.: Benchmarking approximate density functional theory for s/d excitation energies in 3d transition metal cations. J. Comput. Chem. 29, 185 (2008)
Zhao, Y., Truhlar, D.: The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215 (2008)
Hay, H., Wadt, W.: Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270 (1985)
Woon, D., Dunning, T.: Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 98, 1358 (1993)
Ivanova, B., Spiteller, M.: Physical optical properties and crystal structures of organic 5-sulfosalicylates—Theoretical and experimental study. J. Mol. Struct. 1003, 1 (2011)
Office Program Package. http://de.openoffice.org
Stephens, P., McCann, D., Cheeseman, J., Frisch, M.: Determination of absolute configurations of chiral molecules using ab initio time-dependent density functional theory calculations of optical rotation: how reliable are absolute configurations obtained for molecules with small rotations? Chirality 17, S52 (2005)
Stephens, P., Devlin, F., Cheeseman, J., Frisch, M., Bortolini, O., Besse, P.: Determination of absolute configuration using ab initio calculation of optical rotation. Chirality 15, S57 (2003)
Yildiz, A., Selvin, P.: Fluorescence imaging with one nanometer accuracy: application to molecular motors. Acc. Chem. Res. 38, 574 (2005)
Kelley, C.: Iterative Methods for Optimization, SIAM Frontiers in Applied Mathematics. 18, (1999)
Madsen, K., Nielsen, H., Tingleff, O.: Informatics and Mathematical Modelling, 2nd edn. DTU Press, Denmark (2004)
Marquardt, D.: An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 11, 431 (1963)
Lamshoeft, M., Ivanova, B., Spiteller, M.: Chemical identification and determination of sulfonamides in n-component solid mixtures within THz-region—Solid-state Raman spectroscopic and mass spectrometric study. Talanta 85, 2562 (2011)
Ivanova, B., Spiteller, M.: On the chemical identification and determination of flavonoids in solid-state. Talanta. 30(94), 9–21 (2012)
Ivanova, B., Spiteller, M.: Quantitative analysis of solid binary mixtures-vibrational spectroscopy of β-lactam antibiotics within THz-region. J. Pharmaceut. Biomed. Anal. 2012. doi:10.1016/j.jpba.2011.10.028
Acknowledgments
The authors thank the Deutscher Akademischer Austausch Dienst (DAAD), the Deutsche Forschungsgemeinschaft (DFG), the central instrumental laboratories for structural analysis at University of Dortmund (Germany) and the analytical and computational laboratories at the Institute of Environmental Research (INFU) at same University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ivanova, B., Spiteller, M. Novel pyrrolo-quinazolino-quinoline analogues of the natural alkaloids and their inclusion molecular complexes in the native cyclodextrins: experimental versus theoretical study. J Incl Phenom Macrocycl Chem 76, 87–98 (2013). https://doi.org/10.1007/s10847-012-0176-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0176-y