Abstract
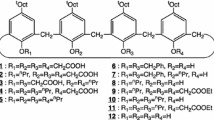
Tetraallyloxy and tetrabenzyloxy derivatives of calix[4]arenes in cone and 1,3-alternate conformations were synthesised and their capacity to extract thallium(I) and silver(I) ions was investigated. ‘Low’-temperature single crystal X-ray structure determinations were recorded for two derivatives in which the calixarene conformation was that of an alternating cone, the aromatic rings lying closely quasi-parallel to the \( \overline{4} \)-axis of the cone. The structure of a tetraallyloxy derivative in the cone conformation was also determined in which a molecule of acetonitrile was included within the calixarene cavity.




Similar content being viewed by others
Notes
Calixarene nomenclature follows that recommended by IUPAC. See: Favre et al. [36].
References
Gutsche, C.D.: Calixarenes: An Introduction, 2nd edn. RSC, Cambridge (2008)
Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J. (eds.): Calixarenes 2001. Kluwer Academic Press, Dordrecht (2001)
Mandolini, L., Ungaro, R. (eds.): Calixarenes in Action. Imperial College Press, London (2000)
Vicens, J., Harrowfield, J. (eds.): Calixarenes in the Nanoworld. Springer, Dordrecht (2006)
Homden, D.M., Redshaw, C.: The use of calixarenes in metal-based catalysis. Chem. Rev. 108(12), 5086–5130 (2008). doi:10.1021/cr8002196
Sliwka-Kaszynska, M.: Calixarenes as stationary phases in high performance liquid chromatography. Crit. Rev. Anal. Chem. 37(3), 211–224 (2007). doi:10.1080/10408340701244672
Creaven, B.S., Donlon, D.F., McGinley, J.: Coordination chemistry of calix[4]arene derivatives with lower rim functionalisation and their applications. Coord. Chem. Rev. 253(7–8), 893–962 (2009). doi:10.1016/j.ccr.2008.06.008
Shokova, E., Kovalev, V.: Calixarene-based anionic receptors. Russ. J. Org. Chem. 45(9), 1275–1314 (2009)
El Nashar, R.M., Wagdy, H.A.A., Aboul-Enein, H.Y.: Applications of calixarenes as potential ionophores for electrochemical sensors. Curr. Anal. Chem. 5(3), 249–270 (2009)
Kimura, K., Tatsumi, K., Yokoyama, M., Ouchi, M., Mocerino, M.: Remarkable thallium(I) selectivity for ion sensors based on π-coordination of calix[4]arene neutral carriers. Anal. Commun. 36(6), 229–230 (1999)
Yoshioka, N., Yajima, S., Kimura, K.: Silver ion sensing using π-coordinate aromatic neutral carriers for soft metal ions. Bunseki Kagaku 52(9), 689–694 (2003)
Harrowfield, J.M., Mocerino, M., Peachey, B.J., Skelton, B.W., White, A.H.: Rare-earth-metal solvent extraction with calixarene phosphates. J. Chem. Soc.-Dalton Transact. (8), 1687–1699 (1996)
Ohto, K.: Review of the extraction behavior of metal cations with calixarene derivatives. Solvent Extr. Res. Dev. Jpn. 17, 1–18 (2010)
Mokhtari, B., Pourabdollah, K., Dallali, N.: A review of calixarene applications in nuclear industries. J. Radioanal. Nucl. Chem. 287(3), 921–934 (2011). doi:10.1007/s10967-010-0881-1
Li, Z.Y., Chen, J.W., Liu, Y., Xia, W., Wang, L.Y.: The use of calixarenes in asymmetric catalysis. Curr. Org. Chem. 15(1), 39–61 (2011)
Ouchi, M., Hakushi, T.: Cation binding by thallium(I) selective crown ethers. Coord. Chem. Rev. 148, 171–181 (1996)
Kudo, Y., Usami, J., Katsuta, S., Takeda, Y.: Solvent extraction of silver picrate by 3 m-crown-m ethers (m = 5, 6) and its mono-benzo-derivative from water into benzene or chloroform: elucidation of an extraction equilibrium using component equilibrium constants. Talanta 62(4), 701–706 (2004). doi:10.1016/j.talanta.2003.09.022
Couton, D., Mocerino, M., Rapley, C., Kitamura, C., Yoneda, A., Ouchi, M.: Silver and thallium ion complexation with allyloxycalix[4]arenes. Aust. J. Chem. 52(3), 227–229 (1999)
Cuc, D., Bouguet-Bonnet, S., Morel-Desrosiers, N., Morel, J.-P., Mutzenhardt, P., Canet, D.: Behavior of cesium and thallium cations inside a calixarene cavity as probed by nuclear spin relaxation evidence of cation-π interactions in water. J. Phys. Chem. B 113(31), 10800–10807 (2009)
Ikeda, A., Shinkai, S.: Unusually high ionophoricity of 1,3-alternate-calix[4]arenes: π-Donor participation in the complexation of cations? Tetrahedron Lett. 33(48), 7385–7388 (1992). doi:10.1016/s0040-4039(00)60194-6
Matthews, S.E., Rees, N.H., Felix, F., Drew, M.G.B., Beer, P.D.: Thallium π-cation complexation by calix[4] tubes: Tl-205 NMR and X-ray evidence. Inorg. Chem. 42(3), 729–734 (2003). doi:10.1021/ic025884x
Matthews, S.E., Schmitt, P., Felix, V., Drew, M.G.B., Beer, P.D.: Calix[4]tubes: a new class of potassium-selective ionophore. J. Am. Chem. Soc. 124(7), 1341–1353 (2002). doi:10.1021/ja011856m
Ghidini, E., Ugozzoli, F., Ungaro, R., Karkema, S., El-Gadl, A.A., Reinhoudt, D.N.: Complexation of alkali metal cations by conformationally rigid, stereoisomeric crown ethers: a quantitative evaluation of preorganization. J. Am. Chem. Soc. 112, 6979–6985 (1990)
Park, C., Chun, S.K., Bartsch, R.A.: Effect of conformation on metal ion extraction by calix[4]arene dicarboxylic acids. J. Incl. Phenom. Macrocycl. Chem. 66(1–2), 95–105 (2010). doi:10.1007/s10847-009-9650-6
Shinkai, S., Fujimoto, K., Otsuka, T., Ammon, H.L.: Synthesis and ion selectivity of conformational isomers derived from calix[4]arenes. J. Org. Chem. 57, 1516–1523 (1992)
Tabakci, M.: Synthesis and evaluation of extraction ability of calix[4]-crown-6 cone conformer and its oligomeric analogue. React. Funct. Polym. 58(1), 27–34 (2004). doi:10.1016/j.reactfunctpolym.2003.11.002
Gutsche, C.D., Dhawan, B., Levine, J.A., Hyun No, K., Bauer, L.J.: Calixarenes 9 conformational isomers of the ethers and esters of calix[4]arenes. Tetrahedron 39(3), 409–426 (1983). doi:10.1016/s0040-4020(01)88541-0
Gutsche, C.D., Reddy, P.A.: Calixarenes 25. Conformations and structures of the products of arylmethylation of calix[4]arenes. J. Org. Chem. 56(15), 4783–4791 (1991)
Dijkstra, P.J., Brunink, J.A.J., Bugge, K.E., Reinhoudt, D.N., Harkema, S., Ungaro, R., Ugozzoli, F., Ghidini, E.: Kinetically stable complexes of alkali cations with rigidified calix[4]arenes—synthesis, X-ray structures, and complexation of calixcrowns and calixspherands. J. Am. Chem. Soc. 111(19), 7567–7575 (1989)
Arduini, A., Casnati, A.: Calixarenes. In: Parker, D. (ed.) Macrocycle Synthesis: A Practical Approach, pp. 145–173. Oxford University Press, Oxford (1996)
Sharma, S.K., Alam, I., Gutsche, C.D.: Selective arylmethylation, arylmethenylation and aroylation of mono-p-cyano-methylcalix[4]arene and tetra-p-cyano-methylcalix[4]arene. Synthesis (9), 1089 (1995)
Sheldrick, G.M.: A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008)
Maeda, T., Kimura, K., Shono, T.: Solvent-extraction of silver and thallium picrates with poly-(crown ether)s and bis-(crown ether)s. Fresen. Z. Anal. Chem. 298(5), 363–366 (1979)
Wong, K.H., Ng, H.L.: Complexes of sodium, potassium, rubidium and cesium picrates with bis-crown ethers. J. Coord. Chem. 11(1), 49–55 (1981)
Asfari, Z., Bilyk, A., Bond, C., Harrowfield, J.M., Koutsantonis, G.A., Lengkeek, N., Mocerino, M., Skelton, B.W., Sobolev, A.N., Strano, S., Vicens, J., White, A.H.: Factors influencing solvent adduct formation by calixarenes in the solid state. Org.Biomol. Chem. 2(3), 387–396 (2004). doi:10.1039/b308214h
Favre, H.A., Hellwinkel, D., Powell, W.H., Smith, H.A. Jr., Tsay, S.S.-C. Pure Appl. Chem. 74, 809.(2002)
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Len Lindoy on the occasion of his 75th birthday and in recognition of his great contribution to the field of macrocyclic chemistry.
Rights and permissions
About this article
Cite this article
Chester, R.T., Couton, D., Lobler, R. et al. The extraction of thallium(I) and silver(I) ions with 1,3-alternate calix[4]arene derivatives. J Incl Phenom Macrocycl Chem 71, 471–477 (2011). https://doi.org/10.1007/s10847-011-0010-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-0010-y