Abstract
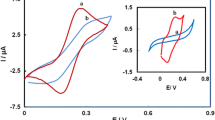
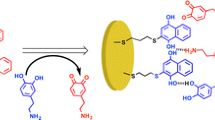
Mixed self-assembled monolayers (SAMs) containing a corrole moiety have been prepared to examine their electrochemical properties and surface acidity, with the eventual goal of biosensor development. Mixed SAMs consisting of 6-mercapto-hexanol (6-MHO) and 8-amino-1-octanethiol (8-AOT) in varying ratios were modified with a free-base corrole and characterized via Osteryoung square-wave voltammetry and contact angle measurements. The surface acidity of the free-base corrole was determined using an electrochemical titration method, with pK a values established at 6.4 when using Fe(CN) 4−6 /Fe(CN) 3−6 as a redox probe and at 6.7 when using iodide, and assigned to the CorH4 + ⇌ CorH3 + H+ equilibrium.




Similar content being viewed by others
References
Gross, Z., Gray, H.B.: How do corroles stabilize high-valent metals? Comment. Inorg. Chem. 27, 61–72 (2006). doi:10.1080/02603590600666256
Gross, Z., Galili, N., Saltsman, I.: The first direct synthesis of corroles from pyrrole. Angew. Chem. Int. Ed. 38, 1427–1429 (1999)
Paolesse, R., Januiqod, L., Nurco, D.J., Mini, S., Sagone, F., Boschi, T., Smith, K.M.: 5,10,15-Triphenylcorrole: a product from a modified Rothemund reaction. Chem. Commun. 1307–1308 (1999). doi:10.1039/a903247i
Gross, Z., Gray, H.B.: Oxidations catalyzed by metallo-corroles. Adv. Synth. Catal. 346, 165–170 (2004). doi:10.1002/adsc.200303145
Meier-Callahan, A.E., DiBilio, A.J., Simkhovich, L., Mahammad, A., Goldberg, I., Gray, H.B., Gross, Z.: Chromium corroles in four oxidation states. Inorg. Chem. 40, 6788–6793 (2001). doi:10.1021/ic010723z
Mahammad, A., Gray, H.B., Meier-Callahan, A.E., Gross, Z.: Aerobic oxidations catalyzed by chromium corroles. J. Am. Chem. Soc. 125, 1162–1163 (2003). doi:10.1021/.ja028216j
Collman, J.P., Kaplum, M., Decréau, R.A.: Metal corroles as electrocatalysts for oxygen reduction. Dalton Trans. 554–559 (2006). doi:10.1039/b512982f
Aviv, I., Gross, Z.: Iron(III) corroles and porphyrins as superior catalysts for the reactions of diazoacetates with nitrogen- or sulfur-containing nucleophilic substrates: synthetic uses and mechanistic insights. Chem. Eur. J. 14, 3995–4005 (2008). doi:10.1002/chem.200701885
Simkhovich, L., Gross, Z.: Iron(IV) corroles are potent catalysts for aziridination of olefins by chloramine-T. Tetrahedron Lett. 42, 8089–8092 (2001). doi:10.1016/S0040-4039(01)01717-8
Barbe, J.-M., Canard, G., Brandès, S., Guilard, R.: Organic–inorganic hybrid sol-gel materials incorporating functionalized cobalt(III) corroles for the selective detection of CO. Angew. Chem. Int. Ed. 117, 3163–3166 (2005). doi:10.1002/ange.200463009
Radecki, J., Stenka, I., Dolusic, E., Dehaen, W., Plavec, J.: Potentiometric discrimination of neutral forms of nitrophenol isomers by liquid membrane isomers incorporated with corroles. Comb. Chem. High-throughput Screen. 7, 375–381 (2004)
Radecki, J., Stenka, I., Dolusic, E., Dehaen, W.: Corroles as receptors in liquid membrane electrodes and their potentiometric response towards salicylic acid. Electrochim. Acta 51, 2282–2288 (2006). doi:10.1016/j.electacta.2005.02.152
Zhang, X.-B., Han, Z.-X., Fang, Z.-H., Shen, G.-L., Yu, R.-Q.: 5,10,15-Tris(pentafluorophenyl)corrole as highly selec-tive neutral carrier for a silver ion-sensitive electrode. Anal. Chim. Acta 562, 210–215 (2006). doi:10.1016/j.aca.2006.01.056
He, C.-H., Ren, F.-L., Zhang, X.-B., Han, Z.-X.: A fluorescent chemical sensor for Hg(II) based on a corrole derivative in a PVC matrix. Talanta 70, 364–369 (2006). doi:10.1016/j.talanta.2006.02.051
Walker, D., Chappel, S., Mahammad, A., Weaver, J.J., Brunschwig, B.S., Winkler, J.R., Gray, H.B., Zaban, A., Gross, Z.: Corrole-sensitized TiO2 solar cells. J. Porphyr. Phthalocyanines 10, 1259–1262 (2006)
Agadjanian, H., Ma, J., Rentsendorj, A., Valluripalli, V., Hwang, J.Y., Mahammad, A., Farkas, D.L., Gray, H.B., Gross, Z., Medina-Kauwe, L.K.: Tumor detection and elimination by a targeted gallium corrole. Proc. Natl. Acad. Sci. USA 106, 6105–6110 (2009). doi:10.1073/pnas.0901531106
Aviv-Harel, I., Gross, Z.: Aura of corroles. Chem. Eur. J. 15, 8382–8394 (2009). doi:10.1002/chem.200900920
Aviv, I., Gross, Z.: Corrole-based applications. Chem. Commun. 1987–1999 (2007). doi:10.1039/b618482k
Szymańska, I., Stobiecka, M., Orlewska, C., Rohand, T., Janssen, D., Dehaen, W., Radecka, H.: Electroactive dipyrro-methene-Cu(II) self-assembled monolayers: complexation reaction on the surface of gold electrodes. Langmuir 24, 11239–11245 (2008). doi:10.1021/la801164f
Szymańska, I., Orlewska, C., Janssen, D., Dehaen, W., Radecka, H.: Dipyrromethene-dodecanethiol self-assembled monolayers deposited onto gold electrodes. Electrochim. Acta 53, 7932–7940 (2008). doi:10.1016/j.electacta.2008.06.002
Kurzątkowska, K., Shpakovsky, D., Radecki, J., Radecka, H., Jingwei, Z., Milaeva, E.: Iron(III) porphyrin bearing 2,6-di-tert-butylphenol pendants deposited onto gold electrodes for amperometric determination of l-histidine. Talanta 78, 126–131 (2009). doi:10.1016/j.talanta.2008.10.051
Kurzątkowska, K., Dolusic, E., Dehaen, W., Sieroń-Stoltny, K., Sieroń, A., Radecka, H.: Gold electrode incorporating corrole as an ion-channel mimetic sensor for determination of dopamine. Anal. Chem. 81, 7397–7405 (2009). doi:10.1021/ac901213h
Jimenez, H.R., Julve, M., Faus, J.: A solution study of the protonation and deprotonation equilibria of 5,10,15,20-tetra(p-sulphonatophenyl) porphyrin. Stability of its magnesium(II), copper(II) and zinc(II) complexes. J. Chem. Soc., Dalton Trans. 1945–1949 (1991). doi:10.1039/DT9910001945
Farjtabar, A., Gharib, F.: Solvent effect on protonation constants of 5,10,15,20-tetrakis(p-sulphonatophenyl) porphyrin in different aqueous solutions of methanol and ethanol. J. Solut. Chem. 39, 231–244 (2010). doi:10.1007/s10953-010-9496-y
Mahammad, A., Weaver, J.J., Gray, H.B., Abdelas, M., Gross, Z.: How acidic are corroles and why? Tetrahedron Lett. 44, 2077–2079 (2003). doi:10.1016/S0040-4039(03)00174-6
Shen, J., Shao, J., Ou, Z., Wenbo, E., Koszarna, B., Gryko, D.T., Kadish, K.M.: Electrochemistry and spectroelectrochemistry of meso-substituted free-base corroles in nonaqueous media: reactions of (Cor)H3, [(Cor)H4]+, and [(Cor)H2]−. Inorg. Chem. 45, 2251–2265 (2006). doi:10.1021/ic051729h
Ou, Z., Shen, J., Shao, J., Wenbo, E., Gałęzowski, M., Gryko, D.T., Kadish, K.M.: Protonated free-base corroles: acidity, electrochemistry, and spectroelectrochemistry of [(Cor)H4]+, [(Cor)H5]2+, and [(Cor)H6]3+. Inorg. Chem. 46, 2775–2786 (2007). doi:10.1021/ic0617893
Kawaguchi, T., Yasuda, H., Shimazu, K.: Electrochemical quartz-crystal microbalance investigation of the reductive desorption of self-assembled monolayers of alkanethiols and mercaptoalkanoic acids on Au. Langmuir 16, 9830–9840 (2000). doi:10.1021/la000756b
Kakiuchi, T., Iida, M., Imabayashi, S., Niki, K.: Double-layer capacitance titration of self-assembled monolayers of ω-functionalized alkanethiols on Au(111) surface. Langmuir 16, 5397–5401 (2000). doi:10.1021/la991358f
Zhao, J., Luo, L., Yang, X., Wang, E., Dong, S.: Determination of surface pK a of SAM using an electrochemical titration method. Electroanalysis 11, 1108–1111 (1999). doi:10.1002/(SICI)1521-4109(199911)11:15<1108:AID-ELAN1108>3.0.CO;2-Z
Smalley, J.F., Chalfant, K., Feldberg, S.W., Nahir, T.M., Bowden, E.F.: An indirect laser-induced temperature jump determination of the surface pK a of 11-mercaptoundecanoic acid monolayers self-assembled on gold. J. Phys. Chem. B 103, 1676–1685 (1999). doi:10.1021/jp983325z
Fears, K.P., Creager, S.E., Latour, R.A.: Determination of the surface pK a of carboxylic and amine-terminated alkane-thiols using surface plasmon resonance spectroscopy. Langmuir 24, 837–843 (2008). doi:10.1021/la701760s
Ju, H., Dai, Z.: Effect of chain length on the surface proper-ties of ω-carboxy alkane thiol self-assembled monolayers. Phys. Chem. Chem. Phys. 3, 3769–3773 (2001). doi:10.1039/b104570a
Saby, C., Ortiz, B., Champagne, G.Y., Bèlanger, D.: Electrochemical modification of glassy carbon electrode using aromatic diazonium salts. 1. Blocking effect of 4-nitrophenyl and 4-carboxyphenyl groups. Langmuir 13, 6805–6813 (1997). doi:10.1021/la961033o
Abiman, P., Crossley, A., Wildgoose, G.G., Jones, J.H., Compton, R.G.: Investigating the thermodynamic causes behind the anomalously large shifts in pK a values of benzoic acid-modified graphite and glassy carbon surfaces. Langmuir 23, 7847–7852 (2007). doi:10.1021/la7005277
Schweiss, R., Pleul, D., Simon, F., Janke, A., Welzel, P.B., Voit, B., Knoll, W., Werner, C.: Electrokinetic potentials of binary self-assembled monolayers on gold: acid-base reactions and double layer structure. J. Phys. Chem. B 108, 2910–2917 (2004). doi:10.1021/jp035724m
Burgess, I., Seivewright, B., Lennox, B.R.: Electric field driven protonation/deprotonation of self-assembled mono-layers of acid-terminated thiols. Langmuir 22, 4420–4428 (2006). doi:10.1021/la052767g
Ngo, T.H., Van Rossom, W., Dehaen, W., Maes, W.: Reductive demetallation of Cu-corroles—a new protective strategy towards functional free-base corroles. Org. Biomol. Chem. 7, 439–443 (2009). doi:10.1039/b819185a
Ngo, T.H., Puntoriero, F., Nastasi, F., Robeyns, K., Van Meervelt, L., Campagna, S., Dehaen, W., Maes, W.: Syn-thetic, structural and photophysical exploration of meso-pyrimidinyl-substituted AB2-corroles. Chem. Eur. J. 16, 5691–5705 (2010). doi:10.1002/chem.201000008
Wang, Q., Zhi, F., Wang, W., Xia, X., Liu, X., Meng, F., Song, Y., Yang, C., Lu, X.: Direct electron transfer of thiol-derivatized tetraphenylporphyrin assembled on gold electrodes in an aqueous solution. J. Phys. Chem. C 113, 9359–9367 (2009). doi:10.1021/jp803725x
Shen, J., Ou, Z., Shao, J., Gałęzowski, M., Gryko, D.T., Kadish, K.M.: Free-base corroles: determination of deprotonation constants in non-aqueous media. J. Porphyr. Phtalocyanines 11, 269–276 (2007)
Acknowledgments
The authors gratefully acknowledge European Union Marie Curie Transfer of Knowledge Research Grants MTKD-CT-2006-042708, the Polish Ministry of Sciences and Higher Education no. 105/6.PR UE/2007/7, and statutory funds of the Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences, Olsztyn, Poland. The authors also thank the IWT (Institute for the Promotion of Innovation through Science and Technology in Flanders) for a doctoral fellowship to Thien H. Ngo and the KU Leuven for continuing financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nulens, W., Grabowska, I., Ngo, T.H. et al. Determination of the surface acidity of a free-base corrole in a self-assembled monolayer. J Incl Phenom Macrocycl Chem 71, 499–505 (2011). https://doi.org/10.1007/s10847-010-9889-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9889-y