Abstract
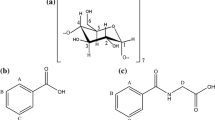
β-cyclodextrin (βCD) and two guests (hexanoic acid and the hydrated fluoride ion) are considered as a system in the NMR chemical shift fast exchange regime. The measured chemical shift variations for the βCD H5 protons are expressed as functions of the mole fractions for the free and complexed states, and their slopes, \(\Delta \delta^{0}_{i} = (\partial \Delta\delta/\partial x_{i})_{xj(j\neq i)}\), are evaluated. When the NaF concentration is varied ([NaF]0/mM = {50,100, 150, 200, 250}) for a defined value of the hexanoic acid concentration ([Hex]0/mM = {5, 7.5, 10, 12.5, 15}), the chemical shift variations of the βCD H5 protons for 2.5 mM βCD solutions in D2O present a second order polynomial variation with a minimum, suggesting two opposing trends. The first trend corresponding to the decrease of Δδ values (relative shielding) is traced to increasingly negative values of \(\Delta \delta^{0}_{\beta{\rm CD} \cdot {\rm Hex\_F^{-}}}\). Corresponding to the increase of Δδ values (relative deshielding), the second trend is mainly associated with the increase of the mole fraction for the inclusion complex βCD·F(H2O) −6 , as the concentration of NaF increases. The kosmotropic, stabilizing features of the fluoride ion are consistent with the results of HF/6–31G* single point energy calculations showing that βCD·F(H2O) −6 is energetically favourable, in contrast with βCD·Cl(H2O) −6 and βCD·Br(H2O) −8 . Due to its smaller size, F(H2O) −6 is not appreciably distorted inside the βCD cavity as compared with Cl(H2O) −6 and Br(H2O) −8 .
Similar content being viewed by others
References
(a) F. Hofmeister: Arch. Exp. Pathol. Pharmakol. 24, 247 (1888); (b) P.H. Hippel and T. Schleich: Acc. Chem. Res. 2, 257 (1969); (c) R.L. Baldwin: Biophys. J. 71, 2056 (1996); (d) M.G. Cacace, E.M. Landau, and J.J. Ramsden: Quart. Rev. Biophys. 30, 241 (1997)
(a) W.R. Saenger: Angew. Chem. Int. Ed. Engl. 19, 344 (1980); (b) W.R. Saenger, J. Jacob, K. Gessler, T. Steiner, D. Hoffmann, H. Sanbe, K. Koizumi, S.M. Smith, and T. Takaha: Chem. Rev. 98, 1787 (1998)
(a) P. Lo Nostro, J.R. Lopes, B.W. Ninham, and P. Baglioni: J. Phys. Chem. B 106, 2166 (2002); (b) N. Funasaki, S. Ishikawa, and S. Neya: J. Phys. Chem. B 107, 10094 (2003)
(a) C. Betzel, W. Saenger, B.E. Hingerty, and G.M. J. Brown: Am. Chem. Soc. 106, 7545 (1984); (b) T. Steiner, W. Saenger, and R.E. Lechner: Molec. Phys. 72, 1211 (1991); (c) T. Steiner and G. Koellner: J. Am. Chem. Soc. 116, 5122 (1994)
(a) Q.X. Guo, Z.Z. Li, T. Ren, X.Q. Zhu, and Y.C.J. Liu: Inclusion Phenom. Mol. Recog. Chem. 17, 149 (1994); (b) H.-J. Schneider, F. Hacket, and V. Rüdiger: Chem. Rev. 98, 1755 (1998); (c) L.D. Wilson and R.E. Verrall: Can. J. Chem. 76, 25 (1998)
S. Lima B.J. Goodfellow J.J.C. Teixeira-Dias (2003) J. Phys. Chem. B 107 14590–14597 Occurrence Handle1:CAS:528:DC%2BD3sXpt1Wqtbk%3D
S. Lima, B.J. Goodfellow, and J.J.C. Teixeira-Dias: J. Phys. Chem. B (2004) (in press)
American Association: J. Am. Diet. Assoc. 100, 1208 (2000)
Y. Matsui M. Ono S. Tokunaga (1997) Bull. Chem. Soc. Jpn 70 535–541 Occurrence Handle1:CAS:528:DyaK2sXhvF2jtL0%3D
M.J. Frisch, G.W. Trucks, H.B. Schlegel, P.M.W. Gill, B.G. Johnson, M.A. Robb, J.R. Cheeseman, T. Keith, G.A. Petersson, J.A. Montgomery, K. Raghavachari, M.A. Al-Laham, V.G. Zakrzewski, J.V. Ortiz, J.B. Foresman, J. Cioslowski, B.B. Stefanov, A. Nanayakkara, M. Challacombe, C.Y. Peng, P.Y. Ayala, W. Chen, M.W. Wong, J.L. Andres, E.S. Replogle, R. Gomperts, R.L. Martin, D.J. Fox, J.S. Binkley, D.J. Defrees, J. Baker, J.P. Stewart, M. Head-Gordon, C. Gonzalez, and A. Pople: Gaussian 94, Revision E.2, Gaussian Inc., Pittsburgh, PA (1995)
H. Gunther (1995) NMR Spectroscopy EditionNumber2 John Wiley & Sons Chichester 339–341
(a) A.M. Moreira da Silva, J. Empis, and J.J.C. Teixeira-Dias: J. Incl. Phenom. Macro. 33, 81 (1999); (b) L. Fielding: Tetrahedron 56, 6151 (2000)
(a) P.W. Hochachka and G.N. Somero: Biochemical Adaptation. Princeton University Press, Princeton, NJ (1984); (b) D.L. Hall and P.L. Darke: J. Biol. Chem. 270, 22697 (1995); (c) P. Terpstra, D. Combes, and A. Zwick: J. Chem. Phys. 92, 65 (1990)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lima, S., Goodfellow, B.J. & Teixeira-dias, J.J.C. Competition between the Hydrated Fluoride Anion and Hexanoic Acid for Inclusion in β-Cyclodextrin: An 1H-NMR Study in Aqueous Solution. J Incl Phenom Macrocycl Chem 54, 35–40 (2006). https://doi.org/10.1007/s10847-005-3489-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10847-005-3489-2