Abstract
In the present work, the host–guest complexation between l-Metheonine/β-Cyclodextrin was investigated using the DFT/B97-D3/6-31G (d) level of the theory. The results obtained clearly indicate that the Orientation B (the carboxylic group of l-Metheonine points toward the primary hydroxyl of β-CD) is energetically favored than that one of the Orientation A (the carboxylic group of l-Metheonine points toward the secondary hydroxyl of β-CD). The energy decomposition analysis, thermodynamic parameters, HOMO, LUMO, the global reactivity descriptors and non covalent interactions-reduced density gradient analysis of the two complexes were calculated and interpreted. The QAIM theory has been used to examine the properties of the bond critical points. Finally, the 1H nuclear magnetic resonance (NMR) chemical shift of the complexes was studied using the Gauge-Including Atomic Orbital (GIAO) method and compared with experimental values.
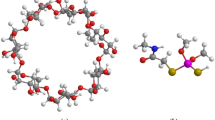
Graphic abstract
The structures of inclusion complexes of l-Metheonine@β-CD.









Similar content being viewed by others
References
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1358 (1997)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Szejtli, J.: Past, present and futute of cyclodextrin research. Pure Appl. Chem. 76(10), 1825 (2004)
Henzi, V., Reichling, D.B., Helm, S.W., MacDermott, A.B.: L-proline activates glutamate and glycine receptors in cultured rat dorsal horn neurons. Mol. Pharmacol. 241, 793 (1992)
Miller, R.A., Buehner, G., Chang, Y., Harper, J.M., Sigler, R., Smith-Wheelock, M.: Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125 (2005)
Lee, W.-J., Hawkins, R.A., Viña, J.R., Peterson, D.R.: Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am. J. Physiol. 274, C1101–C1107 (1998)
Lacey, J.M., Wilmore, D.W.: Is glutamine a conditionally essential amino acid? Nutr. Rev. 48, 297–309 (1990)
Kundu, M., Saha, S., Roy, M.N.: Evidences for complexations of β-cyclodextrin with some amino acids by 1H NMR, surface tension, volumetric investigations and XRD. J. Mol. Liq. 240, 570–577 (2017)
Muddana, H.S., Yin, J., Sapra, N.V., Fenley, A.T., Gilson, M.K.: Blind prediction of SAMPL4 cucurbit[7]uril binding affinities with the mining minima method. J. Comput. Aided Mol. Des. 28, 463–474 (2014)
Antony, J., Sure, R., Grimme, S.: Using dispersion-corrected density functional theory to understand supramolecular binding thermodynamics. Chem. Commun. 51, 1764–1774 (2015)
Safia, H., Ismahan, L., Abdelkrim, G., Mouna, C., Leila, N., Fatiha, M.: Density functional theories study of the interactions between host β-Cyclodextrin and guest 8-Anilinonaphthalene-1-sulfonate: molecular structure, HOMO, LUMO, NBO, QTAIM and NMR analyses. J. Mol. Liq. 280, 218–229 (2019)
Chai, J.-D., Head-Gordon, M.: Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 128, 084106 (2008)
Chai, J.-D., Head-Gordon, M.: Long-range corrected double-hybrid density functionals. J. Chem. Phys. 131, 174105 (2009)
Glendening, E.D., Landis, C.R., Weinhold, F.: Natural bond orbital methods. Wiley Interdiscip. Rev. 2, 1–42 (2012)
Bader, R.F.W., Bader, R.F.: Atoms in Molecules: A Quantum Theory. Clarendon Press, Oxford (1990)
Bader, R.F.: Atoms in molecules. Acc. Chem. Res. 18, 9–15 (1985)
Nowroozi, A., Raissi, H.: Strong intramolecular hydrogen bond in triformylmethane ab initio, AIM and NBO study. J. Mol. Struct. 759, 93–100 (2006)
Hyperchem: Release 7.51 for Windows. Hypercube Inc, Gainesville (2002)
Guendouzi, A., Mekelleche, S.M., Brahim, H., Litim, K.: Quantitative conformational stability host-guest complex of carvacrol and thymol with β-cyclodextrin: a theoretical investigation. J. Incl. Phenom. Macrocycl. Chem. 89, 143–155 (2017)
Pereira, R.A., da Silva Borges, W.M., Peraro, C.R., Anconi, C.P.A.: Theoretical inclusion of deprotonated 2,4-D and dicamba pesticides in ß-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 86, 343–349 (2016)
Boys, S.F., Bernardi, F.: The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision E.01, 2009. Gaussian Inc, Wallingford CT (2013)
Zhou, F., Wang, J., Zhang, Y., Wang, Q., Guo, C., Wang, F., et al.: Comparative studies on the effect of CB[8] on the charge transfer interaction. Theor. Chem. Acc. 138, 50 (2019)
Haiahem, S., Nouar, L., Djilani, I., Bouhadiba, A., Madi, F., Khatmi, D.E.: Host-guest inclusion complex between β-cyclodextrin and paeonol: a theoretical approach. C. R. Chim. 16, 372–379 (2013)
Cheriet, M., Madi, F., Nouar, L., Lafifi, I., Himri, S., Merabet, N., et al.: A DFT study of inclusion complexes of the antituberculosis drugs pyrazinamide and isoniazid with cucurbit[7]uril. J. Incl. Phenom. Macrocycl. Chem. 89, 127–136 (2017)
Senthil Raj, P., Periandy, S., Xavier, S., Attia, M.I.: Molecular structure, vibrational spectra, HOMO, LUMO and NMR studies of methylphenylcyclopropenone based on density functional theories. In: Ebenezar, J. (ed.) Recent Trends in Materials Science and Applications, pp. 655–683. Springer, Cham (2017)
Gümüş, H.P., Tamer, Ö., Avcı, D., Atalay, Y.: Quantum chemical calculations on the geometrical, conformational, spectroscopic and nonlinear optical parameters of 5-(2-Chloroethyl)-2,4-dichloro-6-methylpyrimidine. Spectrochim. Acta Part A 129, 219–226 (2014)
Gümüş, H.P., Tamer, Ö., Avcı, D., Atalay, Y.: Effects of donor–acceptor groups on the structural and electronic properties of 4-(methoxymethyl)-6-methyl-5-nitro-2-oxo-1,2-dihydropyridine-3-carbonitrile. Spectrochim. Acta Part A 132, 183–190 (2014)
Parr, R.G., Pearson, R.G.: Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983)
Geerlings, P., De Proft, F., Langenaeker, W.: Conceptual density functional theory. Chem. Rev. 103, 1793–1874 (2003)
Parr, R.G., Szentpály, L.V., Liu, S.: Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999)
Chattaraj, P.K., Giri, S.: Stability, reactivity, and aromaticity of compounds of a multivalent superatom. J. Phys. Chem. A 111, 11116–11121 (2007)
Padmanabhan, J., Parthasarathi, R., Subramanian, V., Chattaraj, P.K.: Electrophilicity-based charge transfer descriptor. J. Phys. Chem. A 111, 1358–1361 (2007)
Zahedi, E., Shaabani, S., Shiroudi, A.: Following the molecular mechanism of decarbonylation of unsaturated cyclic ketones using bonding evolution theory coupled with NCI analysis. J. Phys. Chem. A 121, 8504–8517 (2017)
Venkataramanan, N.S., Suvitha, A., Kawazoe, Y.: Density functional theory study on the dihydrogen bond cooperativity in the growth behavior of dimethyl sulfoxide clusters. J. Mol. Liq. 249, 454–462 (2018)
Venkataramanan, N.S., Suvitha, A.: Nature of bonding and cooperativity in linear DMSO clusters: a DFT, AIM and NCI analysis. J. Mol. Graph. Model. 81, 50–59 (2018)
Venkataramanan, N.S., Suvitha, A., Kawazoe, Y.: Intermolecular interaction in nucleobases and dimethyl sulfoxide/water molecules: a DFT, NBO, AIM and NCI analysis. J. Mol. Graph. Model. 78, 48–60 (2017)
Reed, A.E., Curtiss, L.A., Weinhold, F.: Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem. Rev. 88, 899–926 (1988)
Behjatmanesh-Ardakani, R., Pourroustaei-Ardakani, F., Taghdiri, M., Kotena, Z.M.: DFT-B3LYP study of interactions between host biphenyl-1-aza-18-crown-6 ether derivatives and guest Cd2 + : NBO, NEDA, and QTAIM analyses. J. Mol. Model. 22, 149 (2016)
Matta, C.F., Boyd, R.J.: The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design. Wiley, Hoboken (2007)
Baerends, E.J., Ziegler, T., Atkins, A.J., Autschbach, J., Baseggio, O., Bashford, D., Bérces, A., Bickelhaupt, F.M., Bo, C., Boerrigter, P.M., Cavallo, L., Daul, C., Chong, D.P., Chulhai, D.V., Deng, L., Dickson, R.M., Dieterich, J.M., Ellis, D.E., van Faassen, M., Fan, L., Fischer, T.H., Fonseca Guerra, C., Franchini, M., Ghysels, A., Giammona, A., van Gisbergen, S.J.A., Goez, A., Götz, A.W., Groeneveld, J.A., Gritsenko, O.V., Grüning, M., Gusarov, S., Harris, F.E., van den Hoek, P., Hu, Z., Jacob, C.R., Jacobsen, H., Jensen, L., Joubert, L., Kaminski, J.W., van Kessel, G., König, C., Kootstra, F., Kovalenko, A., Krykunov, M.V., van Lenthe, E., McCormack, D.A., Michalak, A., Mitoraj, M., Morton, S.M., Neugebauer, J., Nicu, V.P., Noodleman, L., Osinga, V.P., Patchkovskii, S., Pavanello, M., Peeples, C.A., Philipsen, P.H.T., Post, D., Pye, C.C., Ramanantoanina, H., Ramos, P., Ravenek, W., Rodríguez, J.I., Ros, P., Rüger, R., Schipper, P.R.T., Schlüns, D., van Schoot, H., Schreckenbach, G., Seldenthuis, J.S., Seth, M., Snijders, J.G., Solà, M., Stener, M., Swart, M., Swerhone, D., Tognetti, V., te Velde, G., Vernooijs P., Versluis, L., Visscher, L., Visser, O., Wang, F., Wesolowski, T.A., van Wezenbeek, E.M., Wiesenekker, G., Wolff, S.K., Woo, T.K., Yakovlev, A.L.: ADF2017, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands (2007). Accessed 2017. http://www.scm.com
dos Santos Lima, B., de Alcantara Campos, C., da Silva Santos, A.C., Santos, V.C., Trindade, G.D., Shanmugam, S., Pereira, E.W., Marreto, R.N., Duartea, M.C., da Silva Almeidad, J.R., Quintansb, J.D.: Development of morin/hydroxypropyl-β-cyclodextrin inclusion complex: enhancement of bioavailability, antihyperalgesic and anti-inflammatory effects. J. Food Chem. Toxicol. 126, 15–24 (2019)
Schreckenbach, G., Ziegler, T.: Calculation of NMR shielding tensors using gauge-including atomic orbitals and modern density functional theory. J. Phys. Chem. 99, 606–611 (1995)
Pulay, P., Hinton, J. F.: Shielding theory: GIAO method. In: Encyclopedia of Magnetic Resonance. Wiley (2007). https://doi.org/10.1002/9780470034590.emrstm0501
Olah, G.A., Heiner, T., Rasul, G., Prakash, G.K.S.: 1H, 13C, 15N NMR and theoretical study of protonated carbamic acids and related compounds. J. Org. Chem. 63, 7993–7998 (1998)
Bouhadiba, A., Belhocine, Y., Rahim, M., Djilani, I., Nouar, L., Khatmi, D.E.: Host-guest interaction between tyrosine and β-cyclodextrin: molecular modeling and nuclear studies. J. Mol. Liq. 233, 358–363 (2017)
Acknowledgements
This study was supported by the Algerian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nora, M., Ismahan, L., Abdelkrim, G. et al. Interactions in inclusion complex of β-cyclodextrin/l-Metheonine: DFT computational studies. J Incl Phenom Macrocycl Chem 96, 43–54 (2020). https://doi.org/10.1007/s10847-019-00948-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-019-00948-0