Abstract
Purpose
Originally, the coastal region of East Africa was largely covered with coastal forest. With the human settlement, the majority of these forests have been transformed into agricultural land for subsistence farming. Today, only small and geographically isolated forest remnants exist and form a mosaic of different habitat types, including natural, semi-natural and anthropogenic ones. The forest remnants may still represent valuable habitats for typical forest plant and animal species.
Methods
In this study, we surveyed hymenopteran diversity and community composition in different habitat types in southern Kenya. Hereby we considered a small remnant of East African coastal forest, adjoining orchards, shrublands, tree plantations, agricultural fields, and settlements. Hymenoptera represent a large variety of taxa and provide relevant ecosystem services such as pollination to the local people. Hymenoptera were collected with coloured pan traps, identified to family or genus level, and sorted to morphospecies. Habitat parameters such as dead wood, leaf litter, vegetation structure, and the availability of flowers has been assessed for each study site.
Results
In total, we found 419 Hymenoptera individuals of 153 morphospecies. The different habitat types showed distinct species communities of Hymenoptera. Increasing shrub layer and increasing herb cover had a negative effect on hymenopteran species richness and abundance. Flowers, trees, leave litter, and deadwood showed no significant effect on hymenopteran diversity.
Conclusion
Our results underline that small-scale habitat diversity lead to high overall diversity of Hymenoptera. Implications for insect conservation: The coastal forest remnant still holds a unique hymenopteran community with 20 Hymenoptera species exclusively found in this habitat type. It is thus of very high conservation value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human interventions have led to large-scale transformations of natural ecosystems into anthropogenic ecosystems, such as agricultural land and settlements. Thus, natural habitats are increasingly destroyed and the remaining habitats deteriorated due to human activities (Maxwell et al. 2016). This often results in landscapes consisting of a mosaic of different natural, semi-natural and anthropogenic habitat types, with subsequent consequences on biodiversity (Geist and Lambin 2002; Liivamägi et al. 2014). Due to the rapidly increasing human demographic pressure across major parts of Sub-Saharan Africa, there are increasing needs of food and energy resources, and thus many valuable and species-rich habitats are disappearing (Bradshaw et al. 2009). These changes in the habitat composition, habitat quality and landscape structure are often accompanied by changes in species diversity and community composition as well as functionality (Seifert et al. 2022).
Forest habitats in Sub-Saharan Africa are particularly facing challenges due to intense demographic pressure, leading to rapid deforestation and the overexploitation of remaining resources in these habitats (Davis et al. 2020). To illustrate, the majority of East African coastal forests now exist as small and isolated remnants, with many of them being protected for the preservation of their unique biodiversity and cultural significance (Fungomeli et al. 2020). These forest remnants are situated within agricultural landscapes and settlement areas, creating a mosaic-like landscape. In some cases, these last remaining forest fragments serve as crucial habitats for various rare and unique plant and animal species (Luke and Githitho 2003; Wijtten et al. 2011). Additionally, they offer essential resources and ecosystem services to local communities, including wood, water, pollination, and pest control (Losey and Vaughan 2006; Seifert et al. 2022). Despite their importance, there is an ongoing debate about the ability of forest species to persist successfully in these small remnants or if they are at risk of extinction due to stochastic processes (Didham et al. 1996; Melbourne and Hastings 2008).
In this study, we analysed species richness, abundance, diversity and species community composition of Hymenoptera for different habitat types, including a forest remnant, in southern Kenya. We performed a standardized sampling of Hymenoptera in different habitat types to study hymenopteran diversity and community composition. We further analysed potential effects of local habitat parameters for hymenopteran diversity. Based on the data collected, we will answer the following research questions:
-
1.
Does hymenopteran diversity and community composition differ among habitat types, and particularly between the natural forest and anthropogenic habitats?
-
2.
Are there typical forest-associated Hymenoptera, which occur exclusively in the forest remnant?
-
3.
Are tree plantations and orchards potential surrogate habitats for typical forest species?
-
4.
Do local habitat parameters affect species richness and abundance of Hymenoptera?
Material and methods
Study area
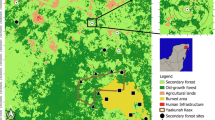
Our study area is located north-east of the city Mombasa (Fig. 1) and includes a patch of natural coastal forest as well as semi-natural and human made ecosystems such as shrubland, orchards, tree plantations, agricultural fields and settlement areas. In the centre of our study area there is a small remnant of dry East African coastal forest (Kaya Kambe forest, 75 ha), which has been preserved until today as this forest is a sacred site for the local community (Fungomeli et al. 2020; Habel et al. 2023). This forest features a still largely closed canopy layer with partly very old and large indigenous tree species (Cynometra suaheliensis, Lannea schweinfurthii, Diospyros kabuyeana, Millettia lasiantha, Markhamia zanzibarica, Gyrocarpus americanus, Sterculia appendiculata), whereby the understory is characterised by numerous shrub species, which indicates a high level of ecosystem disturbances. The forest structure is negatively affected by human activities (Habel et al. 2023), as also observed in other neighbouring forest remnants (Nyamweru 2012; Ngumbau et al. 2020). This forest patch is surrounded by various anthropogenic habitat types, such as orchards, agricultural land, plantations and settlement area (Fig. 1). The forest edge is mostly characterised by a dense shrub layer with single trees. The agricultural land is largely with bare soil (typical for the dry season) or sparsely covered by vegetation (e.g. maize, and few flowering plants in some places). The orchard are dominated by mangoes, coconut palms, bananas, cassava, and cashew trees, but also consist of grassland sites with grazing goats and cattles. Some of the trees were fruiting or flowering while we conducted our field work. Shrubland is dominated by thorny bushes and some coconut palm trees. The understory consists mostly of grasses and partly of bare soil. The tree plantation consists of Eucalyptus globolus with trees of different age and size, and with a dense layer of eucalyptus leaves on the ground. Similarly, also in the coconut palm plantation there is no undergrowth and partly bare soil, with small bushes and palm trees. The settlement area represents small houses, open ground, some few tall trees, and the presence of domestic animals (chickens, goats).
Sampling sites at Kaya Kambe, Kenya. Shown is the study area in southern Kenya (small inlet map) and a detailed overview of the study design, with all study sites located in the following habitat types: East African coastal forest (forest), forest edge, orchard, shrubland, palm plantation, settlement, eucalyptus plantation, and agricultural land
Hymenoptera sampling
As model species we selected Hymenopterans, as representatives of this group rely on very specific imaginal and pre-imaginal (micro-) habitats, and suit therefore as excellent indicators for habitat disturbances, changes in habitat quality and the relevance of specific habitat structures (Lassau and Hochuli 2005). They play a very important role in agriculture such as pollinators and pest control and are therefore of high relevance to the local people and farmers (Gauld and Bolton 1988; Klein et al. 2007). At the same time, they comprise many specialist species which respond sensitive to the deterioration of habitat quality and anthropogenic disturbances (Habel et al. 2018).
Hymenoptera were collected across the different habitat types using pan traps out of three colours (yellow, white, blue, all UV-reflecting colours) from 26.2. to 17.3.2022 (the end of the dry season; see Jaetzold et al. 2012). The set of colours was used to successfully sample as many Hymenoptera as possible, as various hymenopteran species prefer different colours (see Abrahamczyk et al. 2010; Heneberg and Bogusch 2014) and also depending the sex (Campbell and Hanula 2007; Heneberg and Bogusch 2014; Buffington et al. 2021). The three pan traps were attached to a wooden stick at a height of approximately 50 cm (vegetation height). Pan traps were filled with salt solution and detergent (odourless), which is suitable at high evaporation risks and preserves specimens well (Young et al. 2021) and activated for 8 h per day (activation took place between 8am and 6 pm). Sampling was carried out in all habitat types synchronously to guarantee the comparability of data collected. We established in total 166 sampling sites (forest 34, forest edge 17, orchard 35, shrubland 15, palm plantation 5, eucalyptus plantation 14, agricultural land 16, settlement 30) with a minimum distance of 8 m between them. After each round of sampling, trapped individuals were collected and stored in plastic bags (Whirl–Pak® Standard Sterilized Bags).
All captured Hymenoptera were prepared, labelled and identified. The identification for bees and digger wasps was conducted to family and genus level. For the determination to family level we used identification keys of Goulet and Huber (1993), for further determination to genus level for digger wasps we used Bohart and Menke (1976) and for bees we used Eardly et al. (2010). We then grouped individuals into morphospecies (i.e. recognisable taxonomic units) (Krell 2004; Derraik et al. 2002, 2010).
Habitat parameters
Local microhabitat conditions and the availability of specific resources affect the occurrence of organisms (Lassau and Hochuli 2005; Tommasi et al. 2021). Therefore, in addition to habitat type (see above) we also considered local habitat parameters that may influence the occurrence of Hymenoptera. We measured the following parameters for each single sampling site: Availability of dead wood (%), leaf litter on the ground (cm), herb layer (%), shrub layer (%), tree layer (leafs of the vegetation between shrub and canopy) (%), canopy cover (closeness) (%). Percentage values were divided into classes, with 0– < 25%, 25–50%, 50–75% and > 75% and analysed as numeric values (ranging from 1 to 4, respectively). We estimated the number of flowers and classified into the following categories: 0 flower, < 25 flowers, 25–100, > 100 (again coded from 1 to 4). These habitat parameters were collected within a 5 m radius around each set of pan traps.
Statistical analysis
Hill Numbers (Hill 1973) were used to compare diversity across different habitats. As sample frequencies varied between habitats in terms of sites and individuals, iNEXT (v. 3.0.0; Hsieh et al. 2016) was employed to standardise measures using rarefaction and extrapolation. We estimated three measures of diversity qD with increasing consideration of abundance for a standardized sampling intensity endpoint with q = 0 (species richness), q = 1 (the exponential of Shannon’s entropy index), and q = 2 (the inverse of Simpson’s concentration index). This endpoint was set to two times the reference sample size, which is well within the range of reliable extrapolation (Chao et al. 2014). Our standardized diversity estimates represent the diversity measures obtained from a sample size of 30 individuals. Depending on the number of observed samples, the estimates are either obtained via rarefaction or extrapolation techniques.
Non-metric multidimensional scaling (NMDS) based on Bray–Curtis distances was performed to compare the habitats in terms of similarity of species communities. Due to high rates of unique morphospecies and partly low numbers of individuals, the species were pooled per habitat type. Analyses were performed using the metaMDS-function from the “vegan” package version 2.6–4 (Oksanen et al. 2022). The distances were optimized onto two dimensions (k = 2). Stress was ensured to stay below 0.2.
We built two distinct generalized linear models (GLMs) to model species abundance and richness as dependent variables. Given the count-like nature of these variables, we chose a quasipoisson distribution in our models. We preferred the quasipoisson distribution over the Poisson distribution to account for the presence of considerable overdispersion of residuals. Since small distances among some of the sampling sites might compromise non-independence of samples, we plotted Bray–Curtis dissimilarities between pairs of all sites over the respective spatial distances and correlated the two measures. The explanatory variables of the models represented the categories of foliage layer cover, herb layer, shrub layer, tree layer, canopy layer, number of flowers, dead wood cover, and foliage height. To avoid multicollinearity, we tested all pairs of explanatory variables for potential strong correlation. We chose not to include habitat type as main or random effect, since considerable association between the recorded habitat parameters and habitat categories was found (Online Resource 1). Association of the categorical variable habitat types with numerical habitat parameters was evaluated by calculating η2 using the “R” package “heplots” (v. 1.6.2; Friendly et al. 2024). Type II ANOVA with F-tests, which are to prefer in Poisson-models with overdispersion (Faraway 2016), was used to test for significance. All analyses were performed in “R” (version 4.3.1; R Core Team 2023).
Results
A total of 419 specimens of 153 Hymenoptera species and 19 families were collected. Bees (Apidae) and digger wasps (Sphecidae s.l.) represent the largest share, with 24% of all individuals. The orchard harboured most individuals (90 individuals of 46 morphospecies), followed by settlement (88 individuals of 40 morphospecies) and forest (82 individuals of 36 morphospecies). Lowest number of individuals were found in the palm plantation (15 individuals from 12 morphospecies). All data on recorded species and habitat parameters are compiled in Online Resource 2 and Online Resource 3, respectively.
Standardised diversity estimates from rarefaction and extrapolation did not show distinct differences among habitats and ranged between 17.1 species in the settlement area and 22.7 species in the eucalyptus plantation (Table 1). Stronger fluctuations were found in estimates considering abundance, with the lowest values found in the settlement, the forest, and the agricultural land, and highest values in the palm plantation, eucalyptus plantation, and the shrubland (Table 1). Species accumulation curves estimated considerably higher total species numbers for all habitats, and in particular for the eucalyptus plantation, the shrubland, and the orchard (Fig. 2).
Our data indicated that each habitat type had its own species community. One hundred four out of the 153 species could be found in one single study site. This means that 68% of all hymenopteran species captured were only present in one single study site at a time. Eighteen species were recorded in two different study sites and 10 species in three different study sites. In contrast, only two species were present in 25 different sites. The most widespread species thereafter were found in 15 and 11 different sites, respectively. Given that, there was only marginal overlap of species across different habitat types (Table 1, Fig. 3). Twenty species were exclusively found in the forest habitat. These mainly comprised species of the families Bethylidae, Scelionidae, and Diapriidae. Six species of Formicidae (Frmc8, Frmc10, Frmc15, Frmc16, Frmc17, Frmc18) and specimens of the genera Nitela were also largely found in the forest.
Shared and unique morphospecies in the forest habitat and potential surrogates of forest habitats. Given are the numbers of morphospecies and the respective percentage of the total morphospecies count. Numbers disregard the other habitats, i.e., some of the 25 species that occurred in the forest but not in the orchard, the palm plantation, or the eucalyptus plantation, can also occur elsewhere. Numbers below the diagram refer to all other habitats that are not considered here (i.e., settlement area, shrubland, agricultural land, and forest edge)
Likewise, NMDS revealed a distinct species composition and abundance for the forest patch, but also for other investigated habitat types (Fig. 4). The species communities of forest edge, orchard, and eucalyptus plantation, but also of agricultural land were most similar to that of forest. A transition of species communities was observed from these habitats via shrubland and agricultural land towards the coconut palm plantation. The anthropogenic settlement area was likewise clearly separate from other habitats due to its distinctly different species community. While community composition differed substantially among the investigated habitats, overall abundance, observed species, and diversity estimates were not strikingly different. We observed no clear pattern between the more anthropogenically influenced habitats and the natural ones in terms of these measures. The stress value of the NMDS was 0.13.
Comparison of investigated habitats in terms of species composition and abundance by NMDS. Bold black letters indicate habitats: F = forest, E = forest edge, O = orchard, S = settlement area, SL = shrubland, AL = agricultural land, P = palm plantation, and EU = eucalyptus plantation. Light grey acronyms indicate morphospecies; see Online Resource 2 for explanation
Significant effects of habitat parameters were found on the site level. An increasing shrub cover significantly decreased specimen abundance and observed species richness of Hymenoptera. Likewise, increasing herb cover significantly decreased species richness and abundance (Fig. 5). The other parameters studied (tree cover, canopy cover, leaf litter cover, leaf litter height, deadwood cover, and number of flowers), showed no significant relationship with the abundance and richness in this study (Table 2). Indications of spatial autocorrelation among sampling points could not be found (Online Resource 4). No significant correlation between Bray–Curtis dissimilarities and spatial distances was evident in our data (r = 0.01, p = 0.56).
Partial residual plots for the GLMs predicting Hymenoptera abundance and species richness in Kaya Kambe. The plot displays the relationship between the observed dependent variable and the values predicted by the model, with the effects of the other variables removed. A jitter of 0.2 is added to the x- and y-position of the points for better illustration of point densities. A linear regression line with a 95% confidence interval illustrates trends
Discussion
Landscape structure and heterogeneity
The results obtained revealed differences in the species composition depending on the habitat type. With 153 species from 419 individuals caught, we found a high species diversity across the study area. We assume that the species diversity is even higher than estimated as we only sampled taxa during a short period of time, and during the end of the dry season. Diversity will most probably increase in the course of the rainy season, as there is significantly more food sources available for numerous insects at this time and in this region (Habel et al. 2018; Schmitt et al. 2021).
The study area is characterised by a small-scale habitat mosaic, which also promotes species diversity among Hymenoptera (Taki et al. 2008; Szczepko et al. 2020). The different habitats in our study area are strongly interwoven and represents a comparatively heterogeneous and extensively used landscape. Studies show that an intensification of agriculture and an accompanying homogenisation of the landscape configuration may lead to a strong decline in species diversity, as also observed for bees (Gámez-Virués et al. 2015). For Hymenoptera, especially for many digger wasps and bees, the presence of fallow land is of essential relevance, especially for successful development. In contrast, monocultures, the application of pesticides and intense cultivation may lead to rapid decreases in species diversity (Lasway et al. 2022). This was shown in a recent study conducted in the Taita Hills, southern Kenya, with a similar study design. There by far the lowest species diversity of flying insects was found in monocultures, such as eucalyptus plantation (Seifert et al. 2022). In our study, this negative effect was not observed (comparing plantation with natural forest) but standardized species richness was roughly equal among the habitats. Comparatively high values of the Shannon and Simpson diversity, which consider abundance, are attributable to high proportions of singleton species, e.g., in the coconut palm plantation (75%), in the eucalyptus plantation (89%), and in the shrubland (83%), and might mitigate with further sampling. Also, the large number of species that were exclusively found in one single habitat is attributable to the exceptionally high number of species that were only found in a single trap. To some extent, the extensive use may have promoted diversity in the anthropogenic habitats. However, we also assume that the close geographic proximity of the eucalyptus plantation to the natural forest facilitated the dispersal of some species from the neighbouring habitats into monocultures. To scrutinize these trends more closely, much more extensive sampling is required. The high number of individuals caught in the pan traps at the open tree plantations may further be explained by their better visibility, as well as the close geographic proximity. Additionally, some of the forest species were also caught. Previous studies have suggested that good visibility increases the attraction of the traps (Noyes 1989; Wright et al. 2015). Effects on the catchability depending on the visibility, but also the respective colour are also affected by specific light conditions in the habitat type (e.g. forest vs. open agricultural land; see Abrahamczyk et al. 2010).
An interesting observation was made for the sampled proportions of digger wasps and bees, which were the two most frequently sampled taxa. In the forest patch, the proportion of digger wasps caught was high compared to bees. Along the forest edge, we found equal numbers of both, and in the open countryside (with the exception of the settlement area) there were significantly more bees in the pan traps. These shared abundances of digger wasps and bees underline the effect of transition habitats. The digger wasps are predators and feed on other insects and spiders. Bees, on the other hand, feed exclusively on plant products such as pollen and nectar. The habitat selection of both groups is primarily based on the availability of food and nesting opportunities (Witt 1998; Wiesbauer 2017). The least bees were detected in the settlement area and in the forest. These habitats had very few flowers at the time of the survey. The higher number of bees in the palm plantation and in the eucalyptus plantation, despite the very low number of flowers found there, can be attributed to the openness of these habitats (and thus visibility of the pan traps).
Digger wasps, which depend on the presence of other insects and spiders, were rarely found in the plantations and agricultural land. The highest number and proportion of digger wasps were caught in the settlement area. We assume that numerous optimal nesting sites are available on housings and gardens. Houses are made of clay and wood, both materials that are optimal for nesting. In addition, the vegetation density was low and water, required by wasps to build the nest, was available. Many digger wasps need water to build their nests. The most common species of digger wasps caught belonged to the digger wasp genus Trypoxylon (75% of all digger wasps). This genus hunts spiders as provisions for the larvae. Here it can be assumed that these individuals were not attracted by the colour of the shells, which is supposed to imitate flowers, but by the water that Trypoxylon species need to build their nests. The individual brood cells are separated by clay, for which water is needed for production (Bellmann 1995).
Habitat structures
In addition to the general habitat types, certain habitat structures play an even more relevant role than habitat type or landscape structure or the occurrence of specific hymenopteran species (Lassau and Hochuli 2005). Studies showed that explanatory power of the landscape structure but also the habitat parameters strongly influence pollinators, but strongly depending the taxonomic group (Brandt et al. 2017). Our findings on the potential effects of specific habitat parameters on the frequency of Hymenoptera species show that the shrub layer and the herb layer had a negative effect on the abundance and number of species captured. However, the shrub layer probably does not have a negative influence on the Hymenoptera species themselves, but on the methodology applied. As the pan traps were placed at a height of about 50 cm, they were at the level of the shrub layer. We assume that the pan traps attracted significantly fewer individuals as the visibility of the pan traps decreased with the increase in shrub layer. Increased pan trap visibility would also explain why habitats such as the eucalyptus plantation and the settlement show unexpectedly high abundance and diversity of Hymenoptera. In both of these habitats, there was very little undergrowth, so the colour pans were very visible.
As numerous Hymenoptera species, such as bees, visit flowers, one might expect an effect of the number of flowers on diversity measures. However, according to our data and results, the existence of flowers did not significantly affect the prevalence of Hymenoptera. A possible explanation is that bees were attracted by the good visibility of the paint trays over long distances to visit the supposed flowers, as otherwise there was very little flower supply. This could have compensated for the influence of the flowers on the catchability of the traps. Furthermore, due to the fact that there were extremely few flowers at this time of year, comparatively few Hymenoptera that depend on flowers will appear as imagines at this time. It can be assumed that at another time of the year, such as after the rainy season, the composition of the Hymenoptera communities captured will differ significantly from the current one, and especially in the rainy season (when the flower supply increases significantly) flower-visiting Hymenoptera will also occur. Bees are by far the most dependent on flower supply. In this study, bees accounted for 24% of the species. At a time of year when flowers are more abundant, it is likely that the proportion of bees will increase significantly. Our finding contradict with another study showing that the availability of nectar sources strongly drives the occurrence of pollinators in Sub-Saharan smallholder farms (Tommasi et al. 2021).
Conclusion
Our study revealed that the species composition differed markedly between the various habitat types. The natural forest, like the other habitat types, exhibited its own distinctive species community. Some microhabitat structures were found to significantly affect the species community. Despite these striking findings, it is essential to consider certain limitations of our study, including the methodology employed, the landscape structure, and the relatively short time frame considered. The pan traps applied might catch only a fraction of Hymenoptera species, especially the smaller ones enter the traps (see Campbell and Hanula 2007; Hudson et al. 2020). Therefore, further methods such as additional visual trapping with an insect net would be important to generate a more comprehensive data set (Lasway et al. 2022). An additional evaluation of the methodology would be to place the colour trays at different heights, as Hymenoptera fly at different heights due to their different lifestyles and microhabitat preferences. Our study shows that the hymenopteran fauna in the study area is very high, including in monocultures. This might be due to the fact that our study area represents a high level of landscape heterogeneity and a high degree of intersection of the different habitat categories, which drives hymenopteran diversity (Taki et al. 2008; Szczepko et al. 2020) but also a largely uniform distribution of diversity across the area at the landscape level. And, the limited sampling time could also strongly influence the result. The samples were collected over a period of just three weeks. A longer collection period (including both, the dry and the rainy season) could provide further supplementary and important results and findings.
References
Abrahamczyk S, Steudel B, Kessler M (2010) Sampling Hymenoptera along a precipitation gradient in tropical forests: the effectiveness of different coloured pan traps: effectiveness of different coloured pan traps. Entomol Exp Appl 137:262–268
Bellmann H (1995) Bienen, Wespen, Ameisen: Hautflügler Mitteleuropas. Franckh-Kosmos, Stuttgart
Bohart RM, Menke AS (1976) Sphecid wasps of the world: a generic revision. University of California Press, Berkeley
Bradshaw CJ, Sodhi NS, Brook BW (2009) Tropical turmoil: a biodiversity tragedy in progress. Front Ecol Environ 7:79–87
Brandt K, Glemnitz M, Schröder B (2017) The impact of crop parameters and surrounding habitats on different pollinator group abundance on agricultural fields. Agr Ecosyst Environ 243:55–66
Buffington ML, Garretson A, Kula RR, Gates MW, Carpenter R, Smith DR, Kula AAR (2021) Pan trap color preference across Hymenoptera in a forest clearing. Entomol Exp Appl 169:298–311
Campbell JW, Hanula JL (2007) Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J Insect Conserv 11:399–408
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67
Davis KF, Koo HI, Dell’Angelo J, D’Odorico P, Estes L, Kehoe LJ, Kharratzadeh M, Kuemmerle T, Machava D, Pais ADJR, Ribeiro N, Rulli MC, Tatlhego M (2020) Tropical forest loss enhanced by large-scale land acquisitions. Nat Geosci 13:482–488
Derraik JGB, Closs GP, Dickinson KJM, Sirvid P, Barratt BIP, Patrick BH (2002) Arthropod morphospecies versus taxonomic species: a case study with Araneae, Coleoptera, and Lepidoptera. Conserv Biol 16:1015–1023
Derraik JGB, Early JW, Closs GP, Dickinson KJM (2010) Morphospecies and taxonomic species comparison for Hymenoptera. J Insect Sci 10:108
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends Ecol Evol 11:255–260
Eardly C, Kuhlmann M, Pauly A (2010) The bee genera and subgenera of sub-Saharan Africa. Abc Taxa 7:1–145
Faraway JJ (2016) Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models, 2nd edn. CRC Press, Taylor & Francis Group, Boca Raton
Friendly M, Fox J, Monette G (2024) Heplots: visualizing tests in multivariate linear models. R package version 1.6.2. https://CRAN.R-project.org/package=heplots
Fungomeli M, Cianciaruso M, Zannini P, Githitho A, Frascaroli F, Fulanda B, Kibet S, Wiemers M, Mbuvi MT, Matiku P, Chiarucci A (2020) Woody plant species diversity of the coastal forests of Kenya: filling in knowledge gaps in a biodiversity hotspot. Plant Biosyst—Int J Deal Aspects Plant Biol 154:973–982
Gámez-Virués S, Perović DJ, Gossner MM, Börschig C, Blüthgen N, De Jong H, Simons NK, Klein A-M, Krauss J, Maier G, Scherber C, Steckel J, Rothenwöhrer C, Steffan-Dewenter I, Weiner CN, Weisser W, Werner M, Tscharntke T, Westphal C (2015) Landscape simplification filters species traits and drives biotic homogenization. Nat Commun 6:8568
Gauld ID, Bolton B (Eds.) (1988) The hymenoptera. London: Oxford; New York: British Museum (Natural History); Oxford University Press
Geist HJ, Lambin EF (2002) Proximate causes and underlying driving forces of tropical deforestation. Bioscience 52:143
Goulet H, Huber JT (eds) (1993) Hymenoptera of the world: an identification guide to families. Agriculture Canada, Ottawa
Habel JC, Seibold S, Ulrich W, Schmitt T (2018) Seasonality overrides differences in butterfly species composition between natural and anthropogenic forest habitats. Anim Conserv 21:405–413
Habel JC, Schultze-Gebhardt K, Shauri HS, Maarifa AM, Maghenda M, Fungomeli M, Teucher M (2023) Kaya forests: nucleus of cultural and biological diversity and functionality. J Trop Ecol 39:e21
Heneberg P, Bogusch P (2014) To enrich or not to enrich? Are there any benefits of using multiple colors of pan traps when sampling aculeate Hymenoptera? J Insect Conserv 18:1123–1136
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456
Hudson J, Horn S, Hanula JL (2020) Assessing the efficiency of pan traps for collecting bees (hymenoptera: apoidea). J Entomol Sci 55:321–328
Jaetzold R, Schmidt H, Hornet ZB, Shisanya CA (2012) Farm management handbook of Kenya. Volume II: natural conditions and farm information. Eschborn, Nairobi: Ministry of Agriculture/ Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ)
Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B: Biol Sci 274:303–313
Krell F-T (2004) Parataxonomy vs. taxonomy in biodiversity studies—pitfalls and applicability of “morphospecies” sorting. Biodivers Conserv 13:795–812
Lassau SA, Hochuli DF (2005) Wasp community responses to habitat complexity in Sydney sandstone forests. Austral Ecol 30:179–187
Lasway JV, Peters MK, Njovu HK, Eardley C, Pauly A, Steffan-Dewenter I (2022) Agricultural intensification with seasonal fallow land promotes high bee diversity in Afrotropical drylands. J Appl Ecol 59:3014–3026
Liivamägi A, Kuusemets V, Kaart T, Luig J, Diaz-Forero I (2014) Influence of habitat and landscape on butterfly diversity of semi-natural meadows within forest-dominated landscapes. J Insect Conserv 18:1137–1145
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56:311
Luke WRQ, Githitho AN (2003) Biodiversity and the kaya forests. In: Hoorweg J, Muthiga N (eds) Recent advances in coastal ecology: studies from Kenya. Leiden, African Studies Centre, pp 293–301
Maxwell SL, Fuller RA, Brooks TM, Watson JEM (2016) Biodiversity: the ravages of guns, nets and bulldozers. Nature 536:143–145
Melbourne BA, Hastings A (2008) Extinction risk depends strongly on factors contributing to stochasticity. Nature 454:100–103
Ngumbau VM, Luke Q, Nyange M, Wanga VO, Watuma BM, Mbuni YM, Munyao JN, Oulo MA, Mkala EM, Kipkoech S, Itambo M, Hu G-W, Wang Q-F (2020) An annotated checklist of the coastal forests of Kenya, East Africa. PhytoKeys 147:1–191
Noyes JS (1989) A study of five methods of sampling Hymenoptera (Insecta) in a tropical rainforest, with special reference to the Parasitica. J Nat Hist 23:285–298
Nyamweru C (2012) Natural cultural sites of Kenya: changing contexts, changing meanings. J Eastern African Stud 6:270–302
Oksanen J, Simpson GL, Blanchet G, Kindt R, Legendre P, Minchin PR, O´Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista HBA, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill MO, Lahti L, McGlinn D, Ouellette M-H, Ribeiro Cunha E, Smith T, Stier A, Ter Braak CJF, Weedon J (2022) Vegan: community ecology package.
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Schmitt T, Ulrich W, Delic A, Teucher M, Habel JC (2021) Seasonality and landscape characteristics impact species community structure and temporal dynamics of East African butterflies. Sci Rep 11:15103
Seifert T, Teucher M, Ulrich W, Mwania F, Gona F, Habel JC (2022) Biodiversity and ecosystem functions across an afro-tropical forest biodiversity hotspot. Front Ecol Evol 10:816163
Szczepko K, Kruk A, Wiśniowski B (2020) Local habitat conditions shaping the assemblages of vespid wasps (Hymenoptera: Vespidae) in a post-agricultural landscape of the Kampinos National Park in Poland. Sci Rep 10:1424
Taki H, Viana BF, Kevan PG, Silva FO, Buck M (2008) Does forest loss affect the communities of trap-nesting wasps (Hymenoptera: Aculeata) in forests? Landscape vs. local habitat conditions. J Insect Conserv 12:15–21
Tommasi N, Biella P, Guzzetti L, Lasway JV, Njovu HK, Tapparo A, Agostinetto G, Peters MK, Steffan-Dewenter I, Labra M, Galimberti A (2021) Impact of land use intensification and local features on plants and pollinators in sub-saharan smallholder farms. Agric Ecosyst Environ 319:107560
Wiesbauer H (2017) Wilde Bienen: Biologie—Lebensraumdynamik am Beispiel Österreich—Artenporträts. Ulmer, Stuttgart
Wijtten J, Hankinson E, Nuttall M, Gomes I, Lemarkat R (2011) The coastal forest of Shimoni, Kenya: sacred sites as hotspot for biodiversity. In O. Silva, R. Serrano, & R. Chaves (Eds.), VIII International Ethnobotany Symposium. Lisbon; Portugal, p. 617
Witt R (1998) Wespen: beobachten, bestimmen. Naturbuch-Verlag, Augsburg
Wright IR, Roberts SPM, Collins BE (2015) Evidence of forage distance limitations for small bees (Hymenoptera: Apidae). Eur J Entomol 112:303–310
Young RG, Milián-García Y, Yu J, Bullas-Appleton E, Hanner RH (2021) Biosurveillance for invasive insect pest species using an environmental DNA metabarcoding approach and a high salt trap collection fluid. Ecol Evol 11:1558–1569
Acknowledgements
We thank Tobias Seifert, Markus Rainer, Maximilian Schröcker, Nomi Sihler and Hannamariam Mekonnen for help in the field, and Martin Schwarz for his help during species identification. We thank the German Academic Exchange Service (DAAD) for funding this activity in the framework of BIOCULT. Data collection was performed under the NACOSTI research permit NACOSTI/P/22/15886. We thank two anonymous reviewers for critical and very valuable comments on a draft version of this manuscript.
Funding
Open access funding provided by Paris Lodron University of Salzburg. This work is supported by the Deutscher Akademischer Austauschdienst.
Author information
Authors and Affiliations
Contributions
Jonathan Schwarz: Investigation, formal analysis, writing—original draft preparation, writing—review and editing. Jan Christian Habel: Conceptualization, methodology, formal analysis, writing—review and editing. Jonas Eberle: Conceptualization, methodology, formal analysis, writing—review and editing.
Corresponding author
Ethics declarations
Competing interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10841_2024_604_MOESM1_ESM.pdf
Online Resource 1. Association of recorded habitat parameters with investigated habitat types. η and η2 are given within the respective figure panels
Supplementary file1 (PDF 99 KB)
10841_2024_604_MOESM2_ESM.csv
Online Resource 2. Raw data on the collected specimens of Hymenoptera. Individuals were identified to the lowest taxonomic rank possible (ranges family to genus). The identification is given in the column “determination”. The columns “msp” and “abundance” list unique identifiers for morphospecies and their count in the respective sample. The sampling site and associated habitat type is given in columns 1 and 2
Supplementary file2 (CSV 9 KB)
Online resource 3. Raw data of recorded habitat parameters for each sampling site
Supplementary file3 (CSV 5 KB)
10841_2024_604_MOESM4_ESM.pdf
Online resource 4. Scatterplot of pairwise Bray–Curtis dissimilarities over geographical distance. The plot illustrates independence even of closely neighboured sites in terms of species composition. The Pearson correlation coefficient of the variables is r = 0.01 and nor significant (p = 0.56)
Supplementary file4 (PDF 283 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwarz, J., Habel, J.C. & Eberle, J. Effects of habitat biotic features on hymenopteran diversity in East Africa. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00604-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00604-0