Abstract
Insects and arachnids are abundant and diverse, respond to key human impacts and support a diversity of key ecological functions. They are therefore widely recognised as effective surrogates for ecosystem condition. Their efficacy as indicators and surrogates in coastal dunes has, however, rarely been quantified, but might be instructive in guiding management in these often highly impacted coastal ecosystems. In this study, we (1) tested the effects of spatial and habitat drivers on the abundance of key insect and arachnid groups, and (2) used these patterns to identify viable management surrogates for impacts and management actions. We surveyed insect, arachnid and plant assemblages in coastal dunes at 20 sites on the Sunshine Coast in central eastern Australia. We have identified indicators for habitat condition and indicators for impact, and taxa that could be used to measure restoration outcomes. Crucially, five indicator groups also met criteria for being umbrella species, as management actions that increase their abundance would confer benefits to most other insect and arachnid taxonomic groups. We identified seven indicator groups (five insect and two arachnid), and each were affected by multiple spatial and habitat metrics, with metrics quantifying the composition and structure of vegetation communities being the most important predictors of most indicator’s distributions. Implications for insect conservation: We highlight the importance of understanding subtleties in spatial patterns and the unique set of spatial and environmental conditions that each group requires when identifying suitable indicator taxa for use in the conservation and management of coastal dunes.
Similar content being viewed by others
Introduction
Ecosystems are subject to increasing and expanding anthropogenic impacts globally (Vitousek et al. 1997; Halpern et al. 2019). For example, the expansion of human developments result in habitat destruction, degradation and fragmentation, the release of pollutants and the spread of invasive species (Halpern et al. 2019; Siddig et al. 2016). Consequently, there is an increasing desire for management interventions that arrest some of these impacts and recover the ecological condition and associated ecosystem services of damaged ecosystems (Rydgren et al. 2020; Wortley et al. 2013). A desire for expanded restoration action at a global scale (Gilby et al. 2018b; Gillies et al. 2015) is reflected in the United Nations declaration of 2021–2030 as the UN Decade on Ecosystem Restoration (Waltham et al. 2020). However, these important objectives targeting landscape-scale restoration present significant challenges to restoration programs, especially for regions and ecosystems where restoration actions are applied locally, often at small spatial scales by community groups or local governments (Gilby et al. 2021b; Pressey and Bottrill 2009; Rodrigues et al. 2011). Ecologists and natural resource managers must have the capacity to detect and monitor both short- and long-term ecological effects of human induced stressors on ecosystems, and then accurately evaluate the outcomes of management interventions like ecological restoration on ecosystem condition, functioning and/or services (Baldera et al. 2018; González et al. 2014). Identifying appropriate metrics to track such responses is therefore a significant focus in ecology (Hagger et al. 2017; Suding 2011).
Ecological indicators are the concept of using assemblages, species, ecological functions or other small-scale attributes of ecosystems as proxies for overall condition, structure or attributes of the ecosystem as a whole (Hunter et al. 2016; Lindenmayer et al. 2015; Rodrigues and Brooks 2007). They are usually easily measured and monitored surrogates for responses of an ecosystem, community or habitat to environmental stressors (McGeoch 1998, Siddig et al. 2016), and therefore should respond favourably to management interventions (Dale and Beyeler 2001; Hunter et al. 2016) and/or negatively to impacts (Hoffmann and Andersen 2003). Indicator species and umbrella species are well-established ecological surrogates in many ecosystems (Lindenmayer et al. 2015). Umbrellas are typically considered management surrogates (Hunter et al. 2016) and are useful for informing management decisions for conservation (Lindenmayer et al. 2015), environmental and resource management like reserve selection (Rodrigues and Brooks 2007), and for monitoring the success of ecological restoration actions (Sato et al. 2019; Wortley et al. 2013). While indicator species, more generally, are considered indicator surrogates that can be used to monitor changes in diversity, abundance or spatial patterns in response to changes in environmental conditions or impacts (Hunter et al. 2016; Gerlach et al. 2013). The use of indicator species has increased substantially in environmental management over the last decade because of their demonstrated effectiveness across different ecosystems (Gilby et al. 2017; Pander and Geist 2013; Siddig et al. 2016) and spatial scales (Mellin et al. 2011). Therefore, indicator species are increasingly used to track outcomes of actions like restoration (Cristofoli et al. 2010; Fagan et al. 2010; Ottonetti et al. 2006). Indicators are particularly useful in situations where it is not feasible to monitor and measure all organisms within a target ecosystem (Lindenmayer et al. 2014; Senzaki and Yamaura 2016). For example, indicator species may be useful for highly speciose assemblages, or for assemblages with uncertain taxonomy (Petrović 2022). In such instances, selecting cross-taxon surrogates (i.e. a surrogate taxon that reflect diversity or occurrence patterns of another congruent target taxon, as described by Yong et al. (2016) or higher-taxon surrogates (i.e. higher taxa surrogates that reflect the patterns of lower taxonomic levels such as species (e.g. Lovell et al. 2007, Driessen and Kirkpatrick 2019) can help guide biodiversity monitoring and therefore conservation planning and management decisions (e.g. Yong et al. 2016, 2018, Larrieu et al. 2018). While the benefits of indicators are well understood in environmental management, there remain ecosystems, management interventions and impacts for which the efficacy of ecological surrogacy has not been assessed (Grantham et al. 2010; Rodrigues and Brooks 2007), thereby suggesting that such approaches have not been applied as broadly as they could be (Sato et al. 2019).
Coastal environments are ideal for testing the effects of disturbance on ecosystems and to assess the success of subsequent management actions, as they are subject to a diversity of anthropogenic impacts and associated management interventions (Halpern et al. 2019; He et al. 2014). Comprehensive genetic, species and ecosystem records are often limited (McNeill 1994; Ward et al. 1999), so the use of indicator species is appealing for coastal managers seeking to optimise management outcomes (Ward et al. 1999). Coastal dunes are vital ecosystems for a diversity of plant and animal species, including nesting and foraging birds and sea turtles (Baird and Dann 2003; Jones et al. 2007; McLachlan 1991), mammals, reptiles and terrestrial invertebrates (including threatened decapods and insects) (McLachlan & Brown, 2006). However, coastal dunes are negatively impacted by human activities such as urbanisation and trampling (Clark and Johnson 2017; DEWH&A 2008) associated with expanding human populations (Halpern et al. 2019), and so have become reduced in extent, continuity and condition (Martínez et al. 2013). This results in changes to the rates and distributions of key ecological functions (Huijbers et al. 2015), and means that coastal dunes are hotspots for invasive plant (Malavasi et al. 2014) and animal species (Bingham et al. 2018). Therefore, there is a significant and increasing desire globally for coastal dunes to be actively restored via active plantings and the removal of invasive plant species, and/or through the passive removal of trampling effects (Elliott et al. 2022; Martínez et al. 2013). Indicator taxa could be used in coastal dunes to monitor ecosystem condition in response to impacts, and to evaluate the success of management like restoration (González et al. 2014; Suding 2011), but such approaches for this type of ecosystem are uncommon (e.g. Cross et al. 2016; Wünsch et al. 2012).
Insects and arachnids are often considered good ecological indicators as they are found globally in high abundance and diversity, respond quickly to both impacts and management interventions (Andersen and Majer 2004; Barton and Moir 2015; Gerlach et al. 2013) and support a variety of important ecological functions across landscapes (McGeoch 2007; Schowalter 2013). For example, spiders (Arthropoda: Araneae) are key predators that regulate the populations of prey (Gerlach et al. 2013; Pearce and Venier 2006), such as leaf hoppers and frog hoppers (Hemiptera: Auchenorrhyncha) which as sap-sucking insects, play an important role in regulating nutrients and can also directly affect the composition of plant assemblages through herbivory (Moir and Brennan 2007). There are various frameworks that exist to optimise the selection of appropriate indicators and/or surrogates, using systematic approaches, and selection criterion relevant to the conservation or management objective (e.g., Bal et al. 2021, Lindenmayer et al. 2015; McGeoch 1998). Insects (especially Hymenoptera, Coleoptera and Diptera) and arachnids dominate invertebrate assemblages in coastal dunes, and so have significant potential as indicators in these ecosystems (McLachlan 1991; Samways et al. 2020) as abundance is a key criterion for bioindicator selection (McGeoch, 1998). Insects and arachnids that support key ecological functions can be used to monitor ecosystem health and the progress/success of restoration actions (Agosti et al. 2000; Woodcock et al. 2008) (Fig. 1A-D). Ants (Hymenoptera: Formicidae) are ecosystem engineers (Jones et al. 1996) that function as decomposers, seed dispersers, predators (Folgarait 1998). Ants have been used to monitor ecosystem responses to disturbance like fire, mining and urbanisation (Hoffmann and Andersen 2003), changes in ecosystem functioning (Schowalter 2013) and responses of ecosystems to restoration (e.g. Andersen and Majer 2004) in several settings. Pollinators like bees (Hymenoptera: Apidae) might be particularly effective indicators in coastal dunes because a majority of foredune plants are angiosperms requiring pollination by biotic vectors (Devall 1992; Gordon 2000). These functionally important species may also be effective umbrella species as they are often easy to detect and monitor, and managing them would confer benefits to many other species (Fleishman et al. 2001; Gilby et al. 2017; Whiteman and Sites 2008).
Insects perform crucial ecological functions in coastal dunes. Bees (Hymenoptera: Apidae) are important for the pollination of flowering dune species (A), spiders (Arthropoda: Araneae) are key predators (B), Auchenorrhyncha (Hemiptera) are phytophagans and play a role in regulating nutrient cycling (C). Ants (Hymenoptera: Formicidae) are considered ‘ecosystem engineers’ with species that are decomposers and seed dispersers, and others that are predatory (D)
Measuring the success of coastal management programs, including ecological restoration, often involves the assessment of plant assemblages across landscapes (Ruiz-Jaen and Mitchell Aide 2005; Rydgren et al. 2020; Waldén and Lindborg 2016). However, focusing exclusively on the attributes of plant assemblages may miss key components of ecosystems, broader functional responses, and be more time-intensive than using indicator species (Fagan et al. 2010; Hacala et al. 2020; Ruiz-Jaén and Aide 2005). A thorough understanding of how a species or group responds to the structure of ecosystems, key impacts, and the intervening management strategies is crucial for establishing their utility as an indicator species (Hunter et al. 2016; Lindenmayer and Likens 2011). In this sense, establishing how insect assemblages vary along environmental gradients in coastal dunes is vital in properly establishing the efficacy of potential indicators for monitoring and management in this ecosystem, and is another key criterion used in selecting potential indicators (McGeoch, 1998). The aim of this study is to (1) identify how the abundance of key groups of insects and arachnids vary across environmental gradients in coastal dunes, and (2) use these patterns to identify indicators for prioritising, and measuring responses to, management like ecological restoration and other conservation actions (e.g. allocating reserves, managing public activities) in coastal dunes. We test for correlations between components of insect assemblages (e.g. ordinal richness, total insect and arachnid abundance, and the abundance of potential indicator taxa) with attributes of both coastal dune plant assemblages (e.g. plant abundance, cover and size) and the surrounding landscape (e.g. habitat fragmentation and coastal urbanisation). We use pre-existing theories underpinning the selection of ecological surrogates (within the framework of McGeoch, 1998) to identify potential indicator taxa (especially with respect to key ecological functions) or metrics (like species richness, total abundance) within the insect and arachnid assemblage of coastal dunes. In previous studies, insect taxa of the orders Hymenoptera, Diptera and Hemiptera and arachnids (Araneae) have been shown to be important indicators for reflecting spatial and impact gradients (Egerer et al. 2017; Maes and Bonte 2006; Zografou et al. 2017). Therefore, we hypothesised that these taxa would be the most likely indicator candidates, with their abundance tracking key spatial and impact gradients in coastal dunes.
Materials and methods
Study sites
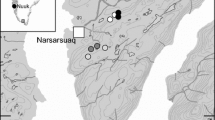
We surveyed coastal dune plant and insect and arachnid assemblages at 20 sites along approximately 40 km of coastline on the Sunshine Coast, Australia (~ 26° 31’S, 153° 5’E to 26° 48’S, 153° 8’E) (Fig. 2). Sites were selected to encompass the gradient of human impacts that exists within the region; from highly urbanised beaches with little to no dune vegetation, to beaches backed by reserves with extensive remnant vegetation (Elliott et al. 2022; Gilby et al. 2021a). A single transect was established at each site that commenced at the high tide line and moved directly into the vegetated dunes (i.e. at an angle perpendicular to the beach). Transects were extended to lengths of either 100 m long or until the first human structure (principally coastal paths and fences) was crossed.
Vegetation surveys
At each transect, we quantified the percent cover and composition of each plant species within 1 m2 quadrats that were positioned every 2 m along the beach and open dune vegetation, and then every 10 m in the forested dunes (Cross et al. 2016; Elliott et al. 2022). At each quadrat in the forested dune, we recorded tree (defined as woody vegetation > 1.5 m high) species and height of trees (using a Suunto PM5/360PC clinometer) intersecting a second transect line positioned at right angles (i.e. parallel to the beach) 5 m either side of the main transect. Tree diameter at breast height was also recorded for all trees surveyed, but correlated with tree height (Pearson’s r = 0.85) and so was not included in any analyses. For further detail and detail on patterns in plant assemblages, see Elliott et al. (2022).
Insect and arachnid surveys
Insect and arachnid assemblages were quantified in 5 × 5 m quadrats positioned along the transect at each site. Here, one quadrat was positioned in the middle of the foredune, with remaining quadrats positioned every 10 m throughout the hind dune (to match with vegetation quadrats described above) (Fig. 2). Sweep nets were used first to collect all flower visiting insects by actively sweeping the entire area of the quadrat. All low-lying, dense shrubs and other vegetation within the quadrat was vacuum sampled using a lithium battery powered blower-vac (Ozito PXC 2 × 18 V), modified with a nylon pool skimmer sock obstructing the air intake to filter insects from the inducted air. All sampling occurred between 0800 and 1200 h to correspond with the activity patterns of insects in coastal dunes and to avoid harsh midday temperatures (Grootaert et al., 2010, Popic et al., 2013). Sampling only occurred during sunny and fine weather with low wind, and sites were sampled in random order to avoid spatial biases. Two sampling events occurred at each site in Summer (January – February) and Spring (September – October) of 2020 to account for changes in flowering composition, climatic conditions and seasonal variability. Insects and arachnids captured using sweep nets were preserved immediately in vials containing 100% ethanol, while vacuum sampled specimens within the skimmer sock were placed into a zip lock bag and frozen prior to subsequent sorting and identification in the laboratory (Krogmann and Holstein 2010). All insects and arachnids were identified to the lowest possible taxonomic order (as per Table S1) and counted in the laboratory under a stereo microscope (Leica M125 C) (Callan et al., 2011). Specimens of each taxa were photographed and kept and stored in 100% ethanol for further taxonomic identification, if necessary.
Distribution of coastal dune insect survey sites across the Sunshine Coast, Australia. The positioning of transects at each site are represented as the purple dots. The map inset displays an example of the transect survey design, including the 1 × 1 m vegetation survey quadrats in yellow squares, and the larger 5 × 5 m quadrats used for insect and arachnid surveys. Insect survey quadrats were positioned with one in the middle of the foredune, and all others spaced approximately 10 m apart in the hind dune (past the initial tree line). The Queensland Spatial Catalogue was used to access spatial layers including the Land use mapping − 1999 to 2013 - South East Queensland NRM dataset to create the urban land layer (orange), the Queensland Palustrine Wetlands dataset to create the palustrine wetland layer (brown) and the Queensland Regional Ecosystems dataset for the natural vegetation layer (green)
Environmental variables
We tested for the effect of a suite of environmental variables on insects and arachnid abundance within each 5 × 5 m quadrat. These can be grouped into either the spatial or habitat attributes of each quadrat (Table 1). Spatial attributes describe the position of each quadrat within the broader context of habitats throughout the region and include measurements of proximity to urbanisation and wetlands. Habitat attributes can be defined into two sub-categories, 1) understorey and 1) canopy layer (trees). Understorey metrics include measures of the percent cover of native grasses, invasive species, total understorey and understorey species richness for each survey quadrat. We included the abundance of two key tree indicator species; Banksia integrifolia and Casuarina equisetifolia identified in the study by Elliott et al. (2022) as key indicators of overall spatial patterns in tree assemblages, along with a measure of overall tree species richness for each quadrat.
We identified collinearity between some of our variables (See Table S2), and subsequently removed all urban and wetland area metrics as sites nearer to urbanisation had lower extents of wetlands nearby (r ≥ 0.7, Dormann et al. 2013). In addition, the abundance of trees and tree species richness (r = 0.83), and tree height and Casuarina equisetifolia abundance (r = 0.73) correlated with each other. Consequently, tree species richness and Casuarina abundance were retained for analyses, and tree height and DBH were removed as insects could not be sampled from vegetation greater than 2 m high.
We selected the seven most abundant and most prevalent insect and arachnid taxa to represent as ‘indicators’ for the overall insect assemblages of coastal dunes. Together these taxa represent over 77% of the total insect and arachnid assemblage, and all were identified at over 44% of quadrats. We also quantified effects of our chosen variables on ordinal richness and total insect and arachnid abundance at each quadrat.
Data analysis
Generalised additive mixed models (GAMMs) in the mgcv package (Wood 2019) of R (R Core Team 2020) were used to explore relationships between variables and insect and arachnid indicator groups. GAMM overfitting was reduced by modelling relationships with three knots or fewer (k = 3). The variable ‘Site’ was included as a random variable in all models, and transect coordinates were added as a smoothed interaction term in each model to account for spatial autocorrelation. Best-fit GAMMs were identified using penalised model selection and Akaike’s Information Criterion (AIC) (Wood 2017). We checked for concurvity between variables in each best fit GAMM, with no concurvity between linear predictor variables identified. Generalised linear models (GLMs) were used to test if indicator taxa could be used as umbrella species by correlating the abundance of each indicator group and the overall taxonomic richness of the assemblage for each quadrat. All analyses were calculated using Tweedie distributions.
Results
Hymenoptera: Formicidae accounted for 21.62% of the total assemblage, and were found in 87.14% of quadrats sampled. Both Hymenoptera and Auchenorrhyncha, were present in 70% of the quadrats, with 442 and 242 individuals collected from each group, respectively. Auchenorrhyncha made up 11.03% of the total assemblage, and Hymenoptera 6.04%. Orthoptera were found in 60% of sampled quadrats, with 189 specimens that accounted for 4.72% of the total assemblage. Dipterans were 26.34% of the total assemblage and were present in 85.71% of quadrats. Similarly, Araneae: Salticidae were found in 71.43% of quadrats, and represented 4.59% of the total assemblage. Araneae: Thomisidae, were found in 60% of sampled quadrats, but made up only 3% of all insect and arachnids surveyed.
Patterns in insect and arachnid ordinal richness and abundance
Coastal dune insect and arachnid ordinal richness was highest in quadrats with higher tree species richness, native grass cover of approximately 30%, nearer to urbanisation and with higher understorey cover (Table 2; Fig. 3A). Total insect and arachnid abundance was highest in quadrats with an understorey species richness of between four and six taxa that are nearer to urbanisation (Table 2; Fig. 3B).
Generalised additive mixed model (GAMM) plots of relationships between (A) insect and arachnid species richness and (B) insect and arachnid abundance (of the entire assemblage; inclusive of 12 orders) and the spatial and habitat variables that best explain the overall patterns, with 95% confidence intervals. The y-axis represents (A) the number of species and (B) abundance
Coastal dune insect and arachnid indicator taxa
Seven groups were selected as potential indicators for the insect and arachnid assemblages of coastal dunes, as these taxa were found in high abundance (between 120 and 1 055 individuals across all quadrats) and occurred in greater than 40% of sampling quadrats across all sites; (1) Hymenoptera: Formicidae, (2) Hymenoptera (bees and wasps, herein Hymenoptera), (3) Hemiptera: Auchenorrhyncha, (4) Orthoptera, (5) Diptera, (6) Aranae: Thomisidae and (7) Aranae: Salticidae.
Formicidae were most abundant in quadrats that were nearest to urbanisation, with low tree species richness and high Casuarina equisetifolia abundance, and understorey cover of between 80 and 90% (Table 2; Fig. 4A). Hymenoptera were most abundant in quadrats approximately 150 m from urbanisation, with both zero native grass (0%) and maximum native grass cover, lowest invasive plant species present and with highest understorey cover (Table 2; Fig. 4B). Auchenorrhyncha were most abundant in quadrats that had native grass cover of approximately 40%, highest understorey cover and understorey species richness of between zero and four species (Table 2; Fig. 4C). Orthoptera show similar patterns to that of Auchenorrhyncha, being in highest abundance in quadrats with native grass cover of approximately 40% and highest understorey cover (Table 2; Fig. 4D). They were also most abundant at sites furthest from wetlands and at sites with greatest tree species richness. In contrast, Diptera were most abundant at quadrats between 1 and 1.5 km from wetlands, and with a total understorey cover of greater than 90% (Table 2; Fig. 4E). Salticidae (Aranae) were most abundant in quadrats further than 50 m from the high tide line (Table 2; Fig. 5B), whereas spiders (Aranae) of the family Thomisidae are in highest abundance at quadrats with both higher tree species richness and total understorey cover (Table 2; Fig. 5C).
Generalised additive mixed model (GAMM) plots of relationships between each insect indicator group and the spatial and habitat variables that best explain the overall patterns, with 95% confidence intervals. This includes (A) Formicidae, (B) Hymenoptera, (C) Auchenorrhyncha, (D) Orthoptera and (E) Diptera. The y-axis represents abundance (number of individuals)
Coastal dune insect and arachnid umbrella taxa
The abundance of five indicator groups were significantly positively correlated with ordinal richness (Fig. 6); Hymenoptera (χ2 < 0.001, p = 0.001), Auchenorrhyncha (χ2 = 0.018, p < 0.01), Orthoptera (χ2 = 0.0014, p < 0.001), Aranae: Thomisidae (χ2 = 0.0017, p < 0.001), and Salticidae (χ2 < 0.001, p = 0.00).
Generalised linear model (GLM) plots for each insect or arachnid umbrella taxa, showing the relationship between abundance and the overall ordinal richness of the entire community. Umbrella taxa include Hymenoptera (bees and wasps), Auchenorrhyncha, Orthoptera, Formicidae, and Araneae: Salticidae. Confidence intervals are 95%. The y-axis represents abundance (number of individuals). SVG image Orthoptera and Formicidae: Tracey Saxby, Integration and Application Network (ian.umces.edu/media-library)
Discussion
Insects and arachnids are a major component of faunal assemblages across a diversity of ecosystems, microhabitats and niches (Kremen et al., 1993) and support crucial ecological functions like detritivory, parasitism, predation, and pollination (Kim, 1993). They are therefore potentially ideal indicator species whose abundance tracks impacts to ecosystems human impacts and/or the outcomes of management actions like ecological restoration (Bazelet and Samways 2011; Fernandes et al. 2019). We found that seven dominant insect and arachnid taxa and the compound metrics of total abundance and ordinal richness correlated significantly with several attributes of both coastal dune ecosystems and the surrounding landscape. Many of these key orders and groups showed similar patterns, thereby demonstrating cross-taxon surrogacy. Therefore, the abundance of each of these taxa could potentially be used as indicators of impacts or management actions that act upon these key attributes of coastal dunes, and as a potential indicator for the other taxa. For example, actions to maximise or restore the cover of native grasses, or total understorey cover on coastal dunes would have benefits for Hymenoptera, Auchenorrhyncha, Orthoptera and Thomisidae (Araneae). Conversely, increasing urbanisation in the coastal zone can increase the abundance of some groups, including Formicidae and Hymenoptera. In highly urban coastal areas, their abundances could potentially be tracked to assess response to management interventions such as invasive plant removal (Barton and Moir 2015; Fiedler et al. 2012). These taxa have been shown in this study to strongly correlate with urbanisation, and therefore may be useful management surrogates for this purpose within this region. Crucially, five of the seven indicator taxa could be potential candidates as umbrella taxa, as managing locations with high abundance of these taxa would confer significant benefits to high diversity of co-occurring taxa (Fleishman et al. 2001). Optimising ecosystem management and monitoring using indicator species or groups relies on establishing their efficacy through thorough spatial analyses across landscapes (Egerer et al. 2017; Gilby et al. 2018a). The results of this study further support the notion that insects are strong indicators of change in coastal landscapes (Longcore 2003), but highlight subtlety in patterns that needs to be considered in selecting indicator groups.
Formicidae are a highly speciose and abundant group of invertebrates that support many key functional roles, and so have been one of the most effective and widely used insect bioindicators (Gerlach et al. 2013; Hoffmann and Andersen 2003). Their use as indicators is underpinned by a thorough understanding of their response to disturbance across multiple spatial scales and relative to multiple pressures (Andersen 2006; Andersen and Majer 2004; Hoffmann and Andersen 2003). In this study, Formicidae in coastal dunes were more abundant at quadrats nearer to urbanisation; a result consistent with many studies across a range of ecosystems where the abundance of Formicidae is higher in more disturbed habitats, and with increased urbanisation (Buczkowski and Richmond 2012, Miguelena and Baker 2019; Rocha and Fellowes 2020; Sanford et al. 2009). Formicidae abundance was also associated with patterns in understorey cover and tree species richness, and the abundance of a key habitat forming tree, coastal she-oak (Casuarina equisetifolia). Lassau and Hochuli (2004) found somewhat similar results in that lower habitat complexity (particularly, lower tree canopy cover) supported higher Formicidae species richness, while Andersen (1986) found more complex habitats (i.e. woodlands) supported high ant species diversity compared to the structurally homogenous heath landscape. Therefore, our results lend further support to the notion that Formicidae are excellent indicators of broad impacts and of management interventions across landscapes (Andersen 2006; Andersen and Majer 2004; Fagan et al. 2010).
Invasive plants have been reported to have both positive and negative effects on insect populations, including Hymenoptera (Davis et al. 2018; Kovács-Hostyánszki et al. 2022; Stephens et al. 2006). In this study, we found a strong negative correlation between bee and wasp (Hymenoptera) abundance and invasive plant cover, and positive correlations between bee and wasp abundance with total understory cover (which is principally native species). Bees and wasps may therefore be useful indicators for the purpose of monitoring the effectiveness of actions removing invasive plants, and their subsequent replacement with native species in coastal dune restoration. Two key weed species dominate the coastal dune ecosystem of this region: asparagus fern (Asparagus aethiopicus) and glory lily (Gloriosa superba) (Elliott et al. 2022). Both produce significant floral resources and can form dense thickets in the understorey and can potentially outcompete native groundcover species (French 2012; Harvey et al. 2013). Ecological restoration programs that target weed removal have been shown previously to significantly increase wild bee abundance and richness (Fiedler et al. 2012; Tonietto and Larkin 2018), however careful consideration is needed to minimise potential negative effects associated with the removal of floral resources (Kovács-Hostyánszki et al. 2022). The goals of restoration programs are often firmly fixed upon improvements in plant community without strong considerations for sympatric taxa such as insects (Tonietto and Larkin 2018; Young 2000). The results of this study support the notion that such narrow foci may result in a lack of quantifying broader functional effects of invasive plant management on species like bees and wasps (Ruiz-Jaen and Mitchell Aide 2005). Different invasive plant species may be expected to have varying levels of impact on insect communities (Kovács-Hostyánszki et al. 2022; Davis et al. 2018), and so different management approaches may need applied for each species that account for their unique life history traits (including peak flowering time) in the planning of restoration and management actions to minimise any potential negative effects associated with removal of floral resources (Kovács-Hostyánszki et al. 2022).
Auchenorrhyncha insects have important functional roles as prey for higher trophic level organisms such as spiders and birds, are hosts for parasitoids (Biedermann et al. 2005), and actively consume plant phloem and xylem, or plant cell contents such as parenchyma thereby modifying vegetation communities (Larivière et al. 2010). Here, coastal dune Auchenorrhyncha were more abundant at sites with dense understorey cover comprising of a maximum of three to four species, and with half of that understorey consisting of native grasses like Spinifex sericeus. Such findings reflect patterns found in other studies (Geppert et al. 2021; Perner et al. 2005). Auchenorrhyncha have been demonstrated to be effective bioindicators for habitat condition and the monitoring of environmental change across a diversity of landscapes, including grasslands (Biedermann et al. 2005; Helbing et al. 2021). This group was also identified as an umbrella species in our analyses, thereby further supporting their potential as indicators in coastal dunes.
Orthoptera are considered effective indicators of habitat condition and quality (Bazelet and Samways 2011) as they are sensitive to changes in microclimate (Borchard et al. 2013; Gardiner and Dover 2008) and vegetation structure (Schirmel et al. 2019). Microclimate conditions such as temperature (Willott and Hassall 1998), light intensity and soil moisture modify site suitability for oviposition (Wünsch et al. 2012), and can impact the hatching and development of orthopterans (Guido and Gianelle 2001). Such physical attributes of ecosystems are significantly affected by changes to the structural characteristics in vegetation communities (Guido and Gianelle 2001; Zografou et al. 2017). In this study, Orthoptera were more abundant at sites with a higher species richness of canopy forming trees, a higher percent cover of understorey plants and with a native grass cover of between 30% and 40%. There could be specific Orthopteran families driving this pattern, such as Tetragoniidae or Gryllidae, that contains species known to inhabit tree species such as Acacia (Otte and Alexander 1983), however, further analyses are required to determine whether these relationships exist in coastal dunes and to identify potential host trees. The positive relationship between Orthopteran abundance and tree species richness is counter to findings of Geppert et al. (2021) and Perner et al. (2005), where plant diversity was not a driver of overall Orthopteran and general arthropod abundance. However, the strong correlation between abundance and high coverage of understorey plants supports the idea that resource availability may have a more important role in driving patterns in the abundance of Orthopterans in coastal dune ecosystems (Geppert et al. 2021; Perner et al. 2005). These patterns also suggest that our Orthoptera assemblage primarily inhabit areas of the dune that are more densely vegetated (i.e. hind dune) (Maes et al. 2006). Orthopterans have been used as bioindicators for management actions (e.g. Borchard et al. (2013), (Alignan et al. 2018); Maes and Bonte (2006) and habitat rehabilitation actions (e.g. Alignan et al. 2018), and restoration primarily in montane heathland (Borchard et al. 2013) and grassland ecosystems (Kenyeres et al. 2020). Therefore, Orthopterans could be an ideal indicators to evaluate the success of management actions in this region, and especially those that aim to increase tree diversity and understorey cover.
While the diversity of Dipteran communities has been well studied across multiple landscapes and ecosystems (Huang et al. 2022; Smith and Mayfield 2015), little information exists on the spatial and habitat drivers that underpin their distribution and abundance within ecosystems (e.g. Engels et al. 2020). In this study, Dipterans were most abundant at quadrants between 1 and 1.5 km from wetlands and with greater understory cover. It is likely that Dipteran groups whose reproductive movements are linked to water bodies (e.g. Culicidae and Chironomidae) (Adler and Courtney 2019; Courtney and Cranston 2015) may be driving these patterns as transitions between water-dwelling larval stages and adult forms affect distributions across landscapes. Management actions aiming to increase the cover of understorey plants at impacted sites may benefit from the use of Dipterans to monitor such changes in habitat structure both within this region and beyond (Grégoire Taillefer and Wheeler 2012).
Spiders are considered excellent indicators of restoration success and broader impacts across a diversity of landscapes (Cristofoli et al. 2010; Hacala et al. 2020; Smith Dicarlo and Debano 2019). For example, attributes of vegetation such as structural complexity and diversity have been shown regularly to correlate with the species richness and abundance of spiders in a variety of settings (Blaise et al. 2022; Damptey et al. 2022; Stokmane and Spungis 2016). However, in this study, jumping spider abundance was only found to be higher at quadrats further from the ocean edge. Jumping spiders include species which have specialised to inhabit tree trunks, bare ground or leaf litter and others that only inhabit grass, herbs or understorey shrubs (Richardson et al. 2006). The effects we found with distance to ocean may reflect these patterns, but further analysis is required to disentangle these effects. Our results suggest that jumping spiders are not indicators for impact, or different metrics of habitat condition. However, they do meet the criteria as an umbrella, and so could be used to assess the success of restoration in degraded sites. Conversely, the abundance Thomisidae was driven entirely by habitat attributes. Thomisidae are mostly ambush predators that inhabit flowers, leaf foliage and leaf litter (Proctor et al. 2015), and so our results may reflect their habitat preferences and/or be a response to high abundance of potential prey species like Auchenorrhyncha or Hymenoptera (bees) that also prefer areas with high understorey cover. Thomisidae are therefore valuable indicators for management interventions that aim to increase understory vegetation cover and tree diversity. Our study therefore shows clear subtlety in the ways in which spider communities respond to impacts across landscapes.
Human pressures have modified the spatial and habitat metrics that insects and arachnids respond to in many settings (Bates et al. 2011; Corcos et al. 2019). The effect of urbanisation on coastal dune insects in this study was varied. For example, Formicidae and total insect and arachnid abundance was higher at quadrats nearer to urbanisation. Conversely, ordinal richness correlated only with habitat metrics associated with the vegetation structure and composition. This pattern may be explained by taxa within the assemblage that may benefit from urbanisation such as Formicidae (e.g. Gibb and Hochuli 2003); Miguelena and Baker (2019); Rocha and Fellowes (2020); Sanford et al. (2009), and Hymenopterans including wasps and bees that can benefit from increased food and nesting resources made available by urban areas (Bates et al. 2011; Kaluza et al. 2016; Theodorou et al. 2020). Formicidae abundance correlated with urbanisation in coastal dune ecosystems in this study; a finding consistent across a range of habitats with increased urbanisation (Buczkowski and Richmond 2012, Miguelena and Baker 2019; Rocha and Fellowes 2020; Sanford et al. 2009). Therefore, in this study, we found that Formicidae and Hymenoptera were best at detecting effects of urbanisation. Certain species within these groups may decline in response to expanding urbanisation (e.g. Miguelena and Baker 2019; Rocha and Fellowes 2020; Sanford et al. 2009), which could potentially lead to shifts in the trophic interactions and functional roles of insect taxa (Christie and Hochuli 2009; Didham et al. 1996), although further research is needed to quantify these effects at the finer spatial resolution of species level interactions within these coastal ecosystems.
Generally, measures of species richness are more commonly used than measures of abundance in assessments of biodiversity (Fleishman et al. 2006). However, there are many challenges to accurately and efficiently quantifying species richness (species counts) in ecosystems, particularly for diverse taxonomic groups (Fleishman et al. 2006). Given that insects and arachnids (Arthropoda) belong to one of the most speciose groups on Earth (Kremen et al. 1993), measurements of species richness of these taxa in the field can often be an under-representation of community diversity (Fleishman et al. 2006). Challenges to accurate quantification of species richness include sampling effort, lack of observer expertise, spatial bias or false positive errors (Johnston et al. 2023). Molecular techniques such as DNA metabarcoding have the potential to overcome these challenges and more accurately identify samples to species level (Watts et al. 2019, Piper et al. 2019, Liu et al. 2020). The application of molecular techniques may also overcome limitations of availability of expert taxonomic knowledge, and time and financial resources for conservation and management programs. Furthermore, there is potential for future research to focus on elucidating which species are influencing the patterns described here, and these may help to guide the selection and use of indicator taxa in monitoring impact and management actions, particularly in coastal ecosystems.
Insect and arachnids are widely recognised as effective indicators of ecosystem condition across a range of habitats (Barton and Moir 2015; Gerlach et al. 2013; Sato et al. 2019). While the benefits of indicator species are well understood in several ecosystems (e.g., grasslands, montane and maritime heathlands), the efficacy of ecological surrogacy has not been thoroughly assessed in many ecosystems, including coastal dunes (Grantham et al. 2010; Rodrigues and Brooks 2007). Here, we support the notion that multiple, complementary umbrella taxa could be valuable for more effectively assessing changes in biodiversity for conservation planning and management (Lambeck 1997, Sattler et al. 2014). Identifying and selecting umbrella taxa that show similar patterns in abundance and presence as congruent taxa may prove more valuable for monitoring site condition and responses to impacts and/or management actions (Fleishman et al. 2000). Adopting a multi-taxon approach may also improve the representation of highly speciose groups (Roberge and Angelstam 2004; Conrad et al. 2007). Coastal conservation and management authorities typically do not have additional resources available for the taxonomic expertise required for accurate monitoring of insect and arachnid umbrellas at the species-level. Therefore, selecting and monitoring the abundances of umbrellas of higher taxonomic groups such as order or family level for these purposes, may reduce the financial and time expenditure for taxonomic expertise, and in some instances provide the opportunity for the involvement of citizen-science based monitoring.
In this study, identifying key insect and arachnid taxa of coastal dune ecosystems was an important first step in generating potential indicator candidates (per McGeoch 1998). Examining taxon responses to spatial and habitat characteristics within these systems, enabled us to identify consistencies in patterns across groups (i.e., cross-taxon surrogacy) to further support their suitability as potential indicators. We show that using the indicator taxa and metrics used in this study would be highly valuable as a tool in coastal conservation and management strategies for monitoring the condition of a site, in response to (1) impacts and (2) targeted management actions, such as restoration, that seek to improve vegetation communities on dunes. We suggest that conservation management models and plans incorporate a multi-taxon approach to selecting insect indicators and umbrellas to help overcome challenges associated with variations in the spatial and habitat requirements of different species, and to best represent entire assemblages. Disentangling subtlety in spatial and habitat condition patterns with key indicators, and understanding the complex ways that each indicator responds to pressures and impacts is crucial in optimising indicator selection in several settings.
Data availability
The authors will provide the data upon reasonable request.
References
Adler PH, Courtney GW (2019) Ecological and Societal Services of Aquatic Diptera. Insects, 10
Agosti D, Majer J, Alonso L, Schultz T (2000) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution, Washington, United States Of America
Alignan J-F, Debras J-F, Dutoit T (2018) Orthoptera prove good indicators of grassland rehabilitation success in the first French natural Asset Reserve. J Nat Conserv 44:1–11
Andersen A (1986) Diversity, seasonality and community organization of ants at adjacent heath and woodland sites in Southeastern Australia. Australian J Zool 34:53–64
Andersen AN (2006) Ants as indicators of restoration success at a uranium mine in tropical Australia. 1, 156–167
Andersen AN, Majer JD (2004) Ants show the way down under: invertebrates as bioindicators in land management. Front Ecol Environ 2:291–298
Baird B, Dann P (2003) The breeding biology of hooded plovers, Thinornis rubricollis, on Phillip Island, Victoria. Emu 103:323–328
Baldera A, Hanson DA, Kraft B (2018) Selecting indicators to monitor outcomes across projects and multiple restoration programs in the Gulf of Mexico. Ecol Ind 89:559–571
Barton P, Moir ML (2015) Invertebrate indicators and ecosystem restoration. In Indicators and Surrogates of Biodiversity and Environmental Change (ed. by D. Lindenmayer, P. Barton & J. Pierson), pp. 59–68. CSIRO Publishing, Victoria, Australia
Bates AJ, Sadler JP, Fairbrass AJ, Falk SJ, Hale JD, Matthews TJ (2011) Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 6:e23459
Bazelet CS, Samways MJ (2011) Identifying grasshopper bioindicators for habitat quality assessment of ecological networks. Ecol Ind 11:1259–1269
Biedermann R, Achtziger R, Nickel H, Stewart AJA (2005) Conservation of Grassland leafhoppers: a brief review. J Insect Conserv 9:229–243
Bingham EL, Gilby BL, Olds AD, Weston MA, Connolly RM, Henderson CJ, Maslo B, Peterson CF, Voss CM, Schlacher TA (2018) Functional plasticity in vertebrate scavenger assemblages in the presence of introduced competitors. Oecologia 188:583–593
Blaise C, Mazzia C, Bischoff A, Millon A, Ponel P, Blight O (2022) Vegetation increases abundances of ground and canopy arthropods in Mediterranean vineyards. Sci Rep 12:3680
Borchard F, Schulte AM, Fartmann T (2013) Rapid response of Orthoptera to restoration of montane heathland. Biodivers Conserv 22:687–700
Buczkowski G, Richmond D-S (2012) The effect of urbanization on ant abundance and diversity: a temporal examination of factors affecting biodiversity. PLOS ONE 7:e41729
Christie FJ, Hochuli DF (2009) Responses of wasp communities to urbanization: effects on community resilience and species diversity. J Insect Conserv 13:213–221
Clark G, Johnston E (2017) Australia State of the Environment 2016: Coasts. An independent report to the Australian Government Minister for Environment and Energy. Canberra: Australian Government Department of the Environment and Energy.
Conrad KF, Fox R, Woiwod IP (2007) In: Alan ICB, Stewart JA, New TR (eds) Monitoring biodiversity: measuring long-term changes in insect abundance. O. T. Lewis, pp 203–225
Corcos D, Cerretti P, Caruso V, Mei M, Falco M, Marini L (2019) Impact of urbanization on predator and parasitoid insects at multiple spatial scales. PLoS ONE 14:e0214068
Courtney GW, Cranston PS (2015) Chapter 40 - Order Diptera. In Thorp and Covich’s Freshwater Invertebrates (Fourth Edition) (ed. by J.H. Thorp & D.C. Rogers), pp. 1043–1058. Academic Press, Boston
Cristofoli S, Mahy G, Kekenbosch R, Lambeets K (2010) Spider communities as evaluation tools for wet heathland restoration. Ecol Ind 10:773–780
Cross SL, Cross AT, Merritt DJ, Dixon KW, Andersen AN (2016) Biodiversity responses to vegetation structure in a fragmented landscape: ant communities in a peri-urban coastal dune system. J Insect Conserv 20:485–495
Dale VH, Beyeler SC (2001) Challenges in the development and use of ecological indicators. Ecol Ind 1:3–10
Damptey FG, Frimpong BF, Debrah DK, Agro PP, Wiafe ED (2022) Vegetation attributes drive the taxonomic richness and functional composition of beetles and spiders in mountainous urban green spaces. Energy Ecol Environ 7:268–280
Davis ES, Kelly R, Maggs CA, Stout JC (2018) Contrasting impacts of highly invasive plant species on flower-visiting insect communities. Biodivers Conserv 27:2069–2085
Devall MS (1992) The biological flora of coastal dunes and wetlands. 2. Ipomoea Pes-Caprae (L.) Roth. J Coastal Res 8:442–456
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends Ecol Evol 11:255–260
Driessen MM, Kirkpatrick JB (2019) Higher taxa can be effective surrogates for species-level data in detecting changes in invertebrate assemblage structure due to disturbance: a case study using a broad range of orders. Austral Entomol 58:361–369
Egerer MH, Arel C, Otoshi MD, Quistberg RD, Bichier P, Philpott SM (2017) Urban arthropods respond variably to changes in landscape context and spatial scale. J Urban Ecol, 3
Elliott BB, Olds AD, Henderson CJ, Rummell AJ, Gilby BL (2022) Optimising restoration and rehabilitation using environmental and spatial drivers of plant assemblages. Landsc Urban Plann 226:104484
Engels S, Medeiros AS, Axford Y, Brooks SJ, Heiri O, Luoto TP, Nazarova L, Porinchu DF, Quinlan R, Self AE (2020) Temperature change as a driver of spatial patterns and long-term trends in chironomid (Insecta: Diptera) diversity. Glob Change Biol 26:1155–1169
Fagan KC, Pywell RF, Bullock JM, Marrs RH (2010) Are ants useful indicators of restoration success in temperate grasslands? Restor Ecol 18:373–379
Fernandes K, van der Heyde M, Coghlan M, Wardell-Johnson G, Bunce M, Harris R, Nevill P (2019) Invertebrate DNA metabarcoding reveals changes in communities across mine site restoration chronosequences. Restor Ecol 27:1177–1186
Fiedler AK, Landis DA, Arduser M (2012) Rapid shift in pollinator communities following invasive species removal. Restor Ecol 20:593–602
Fleishman E, Murphy DD, Brussard PF (2000) A new method for selection of umbrella species for conservation planning. Ecol Appl 10:569–579
Fleishman E, Blair RB, Murphy DD (2001) Empirical validation of a method for umbrella species selection. Ecol Appl 11:1489–1501
Fleishman E, Noss RF, Noon B-R (2006) Utility and limitations of species richness metrics for conservation planning. Ecol Indicators 6(3):543–553
Folgarait PJ (1998) Ant biodiversity and its relationship to ecosystem functioning: a review. Biodivers Conserv 7:1221–1244
French K (2012) Competition strength of two significant invasive species in coastal dunes. Plant Ecol 213:1667–1673
Gardiner T, Dover J (2008) Is microclimate important for Orthoptera in open landscapes? J Insect Conserv 12:705–709
Geppert C, La Bella G, Boscutti F, Sanna F, Marangoni F, Marini L (2021) Effects of temperature and plant diversity on orthopterans and leafhoppers in calcareous dry grasslands. J Insect Conserv 25:287–296
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850
Gibb H, Hochuli DF (2003) Colonisation by a dominant ant facilitated by anthropogenic disturbance: effects on ant assemblage composition, biomass and resource use. Oikos 103:469–478
Gilby BL, Olds AD, Connolly RM, Yabsley NA, Maxwell PS, Tibbetts IR, Schoeman DS, Schlacher TA (2017) Umbrellas can work under water: using threatened species as indicator and management surrogates can improve coastal conservation. Estuar Coast Shelf Sci 199:132–140
Gilby BL, Olds AD, Connolly RM, Henderson CJ, Schlacher TA (2018a) Spatial restoration ecology: placing restoration in a landscape context. Bioscience 68:1007–1019
Gilby BL, Olds AD, Peterson CH, Connolly RM, Voss CM, Bishop MJ, Elliott M, Grabowski JH, Ortodossi NL, Schlacher TA (2018b) Maximizing the benefits of oyster reef restoration for finfish and their fisheries. 19:931–947
Gilby BL, Henderson CJ, Olds AD, Ballantyne JA, Bingham EL, Elliott BB, Jones TR, Kimber O, Mosman JD, Schlacher TA (2021a) Potentially negative ecological consequences of animal redistribution on beaches during COVID-19 lockdown. Biol Conserv 253:108926
Gilby BL, Olds AD, Brown CJ, Connolly RM, Henderson CJ, Maxwell PS, Schlacher TA (2021b) Applying systematic conservation planning to improve the allocation of restoration actions at multiple spatial scales. Restor Ecol 29:e13403
Gillies CL, Fitzsimons JA, Branigan S, Hale L, Hancock B, Creighton C, Alleway H, Bishop MJ, Brown S, Chamberlain D, Cleveland B, Crawford C, Crawford M, Diggles B, Ford JR, Hamer P, Hart A, Johnston E, McDonald T, McLeod I, Pinner B, Russell K, Winstanley R (2015) Scaling-up marine restoration efforts in Australia. 16:84–85
González E, Rochefort L, Boudreau S, Poulin M (2014) Combining indicator species and key environmental and management factors to predict restoration success of degraded ecosystems. Ecol Ind 46:156–166
Gordon DM (2000) Plants as indicators of leafcutter bee (Hymenoptera: Megachilidae) nest habitat in coastal dunes. Pan-Pacific Entomol 76:219–233
Grantham HS, Pressey RL, Wells JA, Beattie AJ (2010) Effectiveness of biodiversity surrogates for conservation planning: different measures of effectiveness generate a kaleidoscope of variation. PLoS ONE 5:e11430
Grégoire Taillefer A, Wheeler TA (2012) Community assembly of Diptera following restoration of mined boreal bogs: taxonomic and functional diversity. J Insect Conserv 16:165–176
Guido M, Gianelle D (2001) Distribution patterns of four Orthoptera species in relation to microhabitat heterogeneity in an ecotonal area. Acta Oecol 22:175–185
Hacala A, Le Roy M, Sawtschuk J, Pétillon J (2020) Comparative responses of spiders and plants to maritime heathland restoration. Biodivers Conserv 29:229–249
Hagger V, Dwyer J, Wilson K (2017) What motivates ecological restoration? Restor Ecol 25:832–843
Halpern BS, Frazier M, Afflerbach J, Lowndes JS, Micheli F, O’Hara C, Scarborough C, Selkoe KA (2019) Recent pace of change in human impact on the world’s ocean. Sci Rep 9:11609–11609
Harvey K, Cherry H, Holland-Clift S, Hargreaves J (2013) Asparagus weeds management manual: current management and control options for asparagus weeds (Asparagus spp.) in Australia. Office of Environment and Heritage (NSW), Sydney
He Q, Bertness MD, Bruno JF, Li B, Chen G, Coverdale TC, Altieri AH, Bai J, Sun T, Pennings SC, Liu J, Ehrlich PR, Cui B (2014) Economic development and coastal ecosystem change in China. Sci Rep 4:5995
Helbing F, Fartmann T, Poniatowski D (2021) Restoration measures foster biodiversity of important primary consumers within calcareous grasslands. Biol Conserv 256:109058
Hoffmann BD, Andersen AN (2003) Responses of ants to disturbance in Australia, with particular reference to functional groups. Austral Ecol 28:444–464
Huang J, Miao X, Wang Q, Menzel F, Tang P, Yang D, Wu H, Vogler AP (2022) Metabarcoding reveals massive species diversity of Diptera in a subtropical ecosystem. Ecol Evol 12:e8535
Huijbers CM, Schlacher TA, Schoeman DS, Olds AD, Weston MA, Connolly RM (2015) Limited functional redundancy in vertebrate scavenger guilds fails to compensate for the loss of raptors from urbanized sandy beaches. Divers Distrib 21:55–63
Hunter M, Westgate M, Barton P, Calhoun A, Pierson J, Tulloch A, Beger M, Branquinho C, Caro T, Gross J, Heino J, Lane P, Longo C, Martin K, McDowell WH, Mellin C, Salo H, Lindenmayer D (2016) Two roles for ecological surrogacy: Indicator surrogates and management surrogates. Ecol Ind 63:121–125
Johnston A, Matechou E, Dennis EB (2023) Outstanding challenges and future directions for biodiversity monitoring using citizen science data. Methods Ecol Evol 14:103–116
Jones CG, Lawton JH, Shachak M (1996) Organisms as Ecosystem Engineers. Ecosystem Management: selected readings. Springer New York, New York, NY, pp 130–147
Jones A, Gladstone W, Hacking N (2007) Australian sandy-beach ecosystems and climate change: ecology and management. Australian Zoologist 34:190–202
Kaluza BF, Wallace H, Heard TA, Klein A-M, Leonhardt SD (2016) Urban gardens promote bee foraging over natural habitats and plantations. Ecol Evol 6:1304–1316
Kenyeres Z, Szabo S, Gábor T, Szinetár C (2020) Orthoptera assemblages as indicators for the restoration of sand grassland networks. North-Western J Zool 16:7–14
Kovács-Hostyánszki A, Szigeti V, Miholcsa Z, Sándor D, Soltész Z, Török E, Fenesi A (2022) Threats and benefits of invasive alien plant species on pollinators. Basic Appl Ecol 64:89–102
Kremen C, Colwell R-K, Erwin T-L, Murphy D-D, Noss R-F, Sanjayan M-A (1993) Terrestrial arthropod assemblages: their use in conservation planning. Conserv Biol 7:796–808
Krogmann L, Holstein J, D (2010) Chap. 18: preserving and specimen handling: insects and other invertebrates. In: Eymann J, Degreef J, Hauser C, Monje JC, Samyn Y, Vanden Spiegel D (eds) Manual on Field Recording techniques and protocols for all Taxa Biodiversity inventories. ABC Taxa, Brussels, Belgium, pp 463–481
Lambeck R-J (1997) Focal species: a multi-species umbrella for nature conservation. Conserv Biol 11:849–856
Larivière MC, Fletcher MJ, Larochelle A (2010) Auchenorrhyncha (Insecta: Hemiptera): catalogue. Fauna New Z, 233
Larrieu L, Gosselin F, Archaux F, Chevalier R, Corriol G, Dauffy-Richard E, Deconchat M, Gosselin M, Ladet S, Savoie J-M, Tillon L, Bouget C (2018) Cost-efficiency of cross-taxon surrogates in temperate forests. Ecol Ind 87:56–65
Lassau SA, Hochuli DF (2004) Effects of habitat complexity on ant assemblages. Ecography 27:157–164
Lindenmayer DB, Likens GE (2011) Direct measurement Versus Surrogate Indicator Species for evaluating environmental change and biodiversity loss. Ecosystems 14:47–59
Lindenmayer DB, Barton PS, Lane PW, Westgate MJ, McBurney L, Blair D, Gibbons P, Likens GE (2014) An empirical assessment and comparison of species-based and habitat-based surrogates: a case study of forest vertebrates and large old trees. PLoS ONE 9:e89807–e89807
Lindenmayer D, Pierson J, Barton P, Beger M, Branquinho C, Calhoun A, Caro T, Greig H, Gross J, Heino J, Hunter M, Lane P, Longo C, Martin K, McDowell WH, Mellin C, Salo H, Tulloch A, Westgate M (2015) A new framework for selecting environmental surrogates. Sci Total Environ 538:1029–1038
Liu M, Clarke L-J, Baker S-C, Jordan G-J, Burridge C-P (2020) A practical guide to DNA metabarcoding for entomological ecologists. Ecol Entomol 45(3):373–385
Longcore T, California USA (2003) Restor Ecol, 11, 397–409
Maes D, Bonte D (2006) Using distribution patterns of five threatened invertebrates in a highly fragmented dune landscape to develop a multispecies conservation approach. Biol Conserv 133:490–499
Maes D, Ghesquiere A, Logie M, Bonte D (2006) Habitat use and mobility of two threatened coastal dune insects: implications for conservation. J Insect Conserv 10:105–115
Malavasi M, Carboni M, Cutini M, Carranza ML, Acosta ATR (2014) Landscape fragmentation, land-use legacy and propagule pressure promote plant invasion on coastal dunes: a patch-based approach. Landscape Ecol 29:1541–1550
Martínez ML, Hesp PA, Gallego-Fernández JB (2013) Coastal Dunes: Human Impact and Need for Restoration. In Restoration of Coastal Dunes (ed. by M.L. Martínez, P.A. Hesp & J.B. Gallego-Fernández). Springer, Berlin, Heidelberg
McGeoch MA (2007) Insects and Bioindication: Theory and Progress. In Insect Conservation Biology: Royal Entomological Society (ed. by A.J.A. Stewart, T.R. New & O.T. Lewis), pp. 144–174. CABI, Wallingford, United Kingdom
McLachlan A (1991) Ecology of coastal dune fauna. J Arid Environ 21:229–243
McLachlan A, Brown AC (2006) Chapter 13 - Coastal Dune Ecosystems and Dune/Beach Interactions. In The Ecology of Sandy Shores (Second Edition) (ed. by A. McLachlan & A.C. Brown), pp. 251–271. Academic Press, Burlington
McNeill SE (1994) The selection and design of marine protected areas: Australia as a case study. Biodivers Conserv 3:586–605
Mellin C, Delean S, Caley J, Edgar G, Meekan M, Pitcher R, Przeslawski R, Williams A, Bradshaw C (2011) Effectiveness of biological surrogates for predicting patterns of marine biodiversity: a global meta-analysis. PLoS ONE 6:e20141–e20141
Miguelena JG, Baker PB (2019) Effects of urbanization on the diversity, abundance, and composition of ant assemblages in an arid city. Environ Entomol 48:836–846
Moir ML, Brennan KEC (2007) Using Bugs (Hemiptera) as Ecological and Environmental Indicators in Forest Ecosystems. In Ecology Research Progress (ed. by S.I. Muñoz), pp. 79–116. Nova Science Publishers, New York
Otte D, Alexander RD (1983) The Australian crickets (Orthoptera: Gryllidae) Academy of Natural Sciences of Philadelphia Monograph, pp 22, 1-147
Ottonetti L, Tucci L, Santini G (2006) Recolonization Patterns of Ants in a rehabilitated Lignite Mine in Central Italy: potential for the Use of Mediterranean ants as indicators of restoration processes. Restor Ecol 14:60–66
Pander J, Geist J (2013) Ecological indicators for stream restoration success. Ecol Ind 30:106–118
Pearce JL, Venier LA (2006) The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: a review. Ecol Ind 6:780–793
Perner J, Wytrykush C, Kahmen A, Buchmann N, Egerer I, Creutzburg S, Odat N, Audorff V, Weisser WW (2005) Effects of plant diversity, plant productivity and habitat parameters on arthropod abundance in montane European grasslands. Ecography 28:429–442
Petrović A (2022) Sizing the knowledge gap in taxonomy: the last dozen years of Aphidiinae Research. Insects, 13
Piper A-M, Batovska J, Cogan N-O-I, Weiss J, Cunningham J-P, Rodoni B-C, Blacket M-J (2019) Prospects and challenges of implementing DNA metabarcoding for high-throughput insect surveillance. GigaScience 8
Pressey RL, Bottrill MC (2009) Approaches to landscape- and seascape-scale conservation planning: convergence, contrasts and challenges. Oryx 43:464–475
Proctor HC, Smith IM, Cook DR, Smith BP (2015) Chapter 25 - Subphylum Chelicerata, Class Arachnida. In Thorp and Covich’s Freshwater Invertebrates (Fourth Edition) (ed. by J.H. Thorp & D.C. Rogers), pp. 599–660. Academic Press, Boston
R Core Team (2020) R: A language and environment for statistical computing. (ed. R Foundation for Statistical Computing). Vienna, Austria
Richardson BJ, Zabka M, Gray MR, Milledge G (2006) Distributional patterns of jumping spiders (Araneae: Salticidae) in Australia. J Biogeogr 33:707–719
Roberge J-M, Angelstam P (2004) Usefulness of the umbrella species concept as a conservation tool. Conserv Biol 18:76–85
Rocha EA, Fellowes MDE (2020) Urbanisation alters ecological interactions: ant mutualists increase and specialist insect predators decrease on an urban gradient. Sci Rep 10:6406
Rodrigues ASL, Brooks TM (2007) Shortcuts for biodiversity conservation planning: the effectiveness of surrogates. Annu Rev Ecol Evol Syst 38:713–737
Rodrigues RR, Gandolfi S, Nave AG, Aronson J, Barreto TE, Vidal CY, Brancalion PHS (2011) Large-scale ecological restoration of high-diversity tropical forests in SE Brazil. For Ecol Manag 261:1605–1613
Ruiz-Jaen MC, Mitchell Aide T (2005) Restoration success: how is it being measured? Restor Ecol 13:569–577
Ruiz-Jaén MC, Aide TM (2005) Vegetation structure, species diversity, and ecosystem processes as measures of restoration success. For Ecol Manag 218:159–173
Rydgren K, Auestad I, Halvorsen R, Hamre LN, Jongejans E, Töpper JP, Sulavik J (2020) Assessing restoration success by predicting time to recovery—but by which metric? J Appl Ecol 57:390–401
Samways MJ, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kaila L, Kwak ML, Maes D, Mammola S, Noriega JA, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Stork NE, Suhling F, Vorster C, Cardoso P (2020) Solutions for humanity on how to conserve insects. Biol Conserv 242:108427
Sanford MP, Manley PN, Murphy DD (2009) Effects of urban development on ant communities: implications for ecosystem services and management. Conserv Biol 23:131–141
Sato CF, Westgate MJ, Barton PS, Foster CN, O’Loughlin LS, Pierson JC, Balmer J, Chapman J, Catt G, Detto T, Hawcroft A, Kavanagh RP, Marshall D, McKay M, Moseby K, Perry M, Robinson D, Schroder M, Tuft K, Lindenmayer DB (2019) The use and utility of surrogates in biodiversity monitoring programmes. J Appl Ecol
Sattler T, Pezzatti GB, Nobis MP, Obrist MK, Roth T, Moretti M (2014) Selection of multiple umbrella species for functional and taxonomic diversity to represent urban biodiversity. Conserv Biol 28:414–426
Schirmel J, Gerlach R, Buhk C (2019) Disentangling the role of management, vegetation structure, and plant quality for Orthoptera in lowland meadows. Insect Sci 26:366–378
Schowalter TD (2013) Insects and sustainability of Ecosystem services, 1st edn. CRC, p 1201b14911
Senzaki M, Yamaura Y (2016) Surrogate species versus landscape metric: does presence of a raptor species explains diversity of multiple taxa more than patch area? Wetlands Ecol Manage 24:427–441
Siddig AAH, Ellison AM, Ochs A, Villar-Leeman C, Lau MK (2016) How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in ecological indicators. Ecol Ind 60:223–230
Smith TJ, Mayfield MM (2015) Diptera species and functional diversity across tropical Australian countryside landscapes. Biol Conserv 191:436–443
Smith Dicarlo LA, Debano SJ (2019) Spider community responses to grassland restoration: balancing trade-offs between abundance and diversity. Restor Ecol 27:210–219
Stephens CJ, Schellhorn NA, Wood GM, Austin AD (2006) Parasitic wasp assemblages associated with native and weedy plant species in an agricultural landscape. Australian J Entomol 45:176–184
Stokmane M, Spungis V (2016) The influence of vegetation structure on spider species richness, diversity and community organization in the Apšuciems Calcareous Fen, Latvia. Anim Biodivers Conserv, 39
Suding KN (2011) Toward an era of restoration in ecology: successes, failures, and opportunities ahead. Annu Rev Ecol Evol Syst 42:465–487
Theodorou P, Radzevičiūtė R, Lentendu G, Kahnt B, Husemann M, Bleidorn C, Settele J, Schweiger O, Grosse I, Wubet T, Murray TE, Paxton RJ (2020) Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat Commun 11:576
Tonietto RK, Larkin DJ (2018) Habitat restoration benefits wild bees: a meta-analysis. J Appl Ecol 55:582–590
Waldén E, Lindborg R (2016) Long term positive effect of grassland restoration on plant diversity - success or not? PLoS ONE 11:e0155836
Ward TJ, Vanderklift MA, Nicholls AO, Kenchington RA (1999) Selecting marine reserves using habitats and species assemblages as surrogates for biological diversity. Ecol Appl 9:691–698
Watts C, Dopheide A, Holdaway R, Davis C, Wood J, Thornburrow D, Dickie I-A (2019) DNA metabarcoding as a tool for invertebrate community monitoring: a case study comparison with conventional techniques. Aust Entomol 58:675–686
Whiteman NK, Sites RW (2008) Aquatic insects as umbrella species for ecosystem protection in Death Valley National Park. J Insect Conserv 12:499–509
Willott SJ, Hassall M (1998) Life-history responses of British grasshoppers (Orthoptera: Acrididae) to temperature change. Funct Ecol 12:232–241
Wood S (2019) Mgcv: mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation. R Package Version 1:8–24
Woodcock BA, Edwards AR, Lawson CS, Westbury DB, Brook AJ, Harris SJ, Brown VK, Mortimer SR (2008) Contrasting success in the restoration of plant and phytophagous beetle assemblages of species-rich mesotrophic grasslands. Oecologia 154:773–783
Wortley L, Hero J-M, Howes M (2013) Evaluating ecological restoration success: a review of the literature. Restor Ecol 21:537–543
Wünsch Y, Schirmel J, Fartmann T (2012) Conservation management of coastal dunes for Orthoptera has to consider oviposition and nymphal preferences. J Insect Conserv 16:501–510
Yong DL, Barton PS, Okada S, Crane M, Lindenmayer DB (2016) Birds as surrogates for mammals and reptiles: are patterns of cross-taxonomic associations stable over time in a human-modified landscape? Ecol Ind 69:152–164
Young TP (2000) Restoration ecology and conservation biology. Biol Conserv 92:73–83
Zografou K, Adamidis GC, Komnenov M, Kati V, Sotirakopoulos P, Pitta E, Chatzaki M (2017) Diversity of spiders and orthopterans respond to intra-seasonal and spatial environmental changes. J Insect Conserv 21:531–543
Acknowledgements
The authors acknowledge Sunshine Coast Council and University of the Sunshine Coast for funding this project. The authors thank Felicity Osborne, Jesse Mosman, Taylor Cooper, Ashley Rummell, Nicholas Ortodossi, Cody James, Lucy Goodridge Gaines, Hannah Perry, and Sarah Thackwray for their assistance in the field and Ashleigh Miller, Josh Hill, Katie Jee and Amber Forbes for volunteering their time to assist us in processing samples in the lab.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors acknowledge the Sunshine Coast Council and University of the Sunshine Coast for funding this project.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
CRediT authorship contribution statementBrittany Elliott: Conceptualization, Methodology, Investigation, Formal Analysis, Writing – Original draft. Alison Shapcott: Conceptualization, Methodology, Writing – Reviewing and Editing. Christopher Henderson: Conceptualization, Methodology, Writing – Reviewing and Editing. Andrew Olds: Conceptualization, Methodology, Writing – Reviewing and Editing. Ben Gilby: Conceptualization, Methodology, Formal Analysis, Writing – Reviewing and Editing and Supervision.
Corresponding author
Ethics declarations
Ethical approval
The collection of insects and arachnids in this study was permitted under a Sunshine Coast Council Activity Permit.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elliott, B.B., Shapcott, A., Henderson, C.J. et al. Identifying insect and arachnid indicator taxa for impacts and management in coastal landscapes. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00580-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00580-5