Abstract
Studies on the effects of grazing disturbances in grasslands have shown mixed results for spider diversity, mainly regarding their guilds. While ungrazing, low, and moderate grazing potentially enhance the diversity of orb-weavers in spider communities, heavy grazing seems to reduce species’ richness. On the population level though, studies of orb-weavers are scarce, and the effects of grazing in natural grasslands are unknown. In this way, we investigated the effects of different grazing levels on population persistence of orb-weaver spiders, hypothesizing that low to intermediate disturbances benefit populations. We predict that high grazing, due to the removal of vegetation structure, will negatively affect the occupancy and abundance of orb-weavers. For that, we experimentally controlled grazing pressure and obtained population occurrence and counts of two orb-weaver spider species, Argiope argentata and Alpaida quadrilorata. We found that A. argentata was negatively affected by grazing, as it relies on higher vegetation for web-building. In contrast, A. quadrilorata, which occurs in cattle-resistant rosette plants, showed no effects of grazing. Implications for insect conservation: Our study emphasizes the need for balanced grazing practices and habitat conservation to protect orb-weaver spiders and other arthropods, as well as species-specific effects for species from the same guild, underscoring their ecological significance in maintaining ecosystem stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generalist predators are midranking carnivorous that vary in many shapes and sizes, playing an important role in regulating, mainly herbivores (Gagnon et al. 2019; Macé et al. 2019; Michalko et al. 2019; Wray et al. 2021). Such predators usually occur sympatrically and have similar needs of food and energy intakes, as well as broader diets than top predators (Lesmeister et al. 2015; Whitney et al. 2018; Wray et al. 2021), providing them superior resilience to disturbances (Wimp et al. 2019). However, generalist predators are largely affected by chronic anthropogenic disturbances (Rito et al. 2017; Antongiovanni et al. 2020) such as agriculture and livestock, since these activities may threaten them through human conflict, environmental degradation and declines in prey population (Wang et al. 2015; Newsome et al. 2017; Wimp et al. 2019).
Among arthropod generalist predators, spiders have been one of the main focus in studies of grazing chronic disturbance (Macé et al. 2019; Filazzola et al. 2020). Despite some inconclusive results regarding the effects of grazing on spiders inhabiting natural grasslands (Silva and Ott 2017; Muvengwi et al. 2018; Samu et al. 2018), results usually follow a trend of intermediate disturbance (Wang and Tang 2019; Oyarzabal and Guimarães 2021). Ungrazing, low and moderate grazing usually enhance spider diversity (Szmatona-Túri et al. 2018; Ferreira et al. 2020; Oyarzabal and Guimarães 2021), while heavy grazing reduces species richness and abundance (Szmatona-Túri et al. 2018; Hashemi et al. 2019; Oyarzabal and Guimarães 2021). In addition, grazing pressure seems to affect spider community composition, without homogenization but rather a species turnover within guilds and families (Dennis et al. 2015; da Silva Bomfim et al. 2021; Oyarzabal and Guimarães 2021). Hence, grazing disturbance seems to affect spider guilds in opposite ways, where diversity of ground-dweller spiders may be enhanced while orb-weaver spiders appear to be particularly sensitive to vegetation removal (Nogueira and Pinto-da-Rocha 2016; Neilly et al. 2020), losing species richness in heavy grazed environments (Oyarzabal and Guimarães 2021).
The removal of above-ground plant biomass provoked by chronic grazing (Tälle et al. 2016; Pett and Bailey 2019; Ferreira et al. 2020; da Silva Bomfim et al. 2021) directly affects the primary hunting strategy of orb-weavers, the ability to build webs using the tridimensional vegetal structure (Nogueira and Pinto-da-Rocha 2016). Without physical structures to build their web, spiders are unable to find prey (Torma et al. 2019; Helden et al. 2020; Fischer et al. 2021) and mates (Cory and Schneider 2018; Weiss and Schneider 2021), as well as avoid predation (Blackledge and Wenzel 1999; da Silva Bomfim et al. 2021; Narimanov et al. 2021). Consequently, the simplification of habitat structure induced by grazing can culminate in the exclusion of orb-weaver species from grasslands (Oyarzabal and Guimarães 2021). However, the emphasis on the literature has remained on the orb-weavers community level (Rodrigues et al. 2009; da Silva Bomfim et al. 2021), leaving a notable gap in understanding how populations respond to the persistent disturbances induced by grazing.
In this way, our objective was to assess the effects of grazing pressure on populations of two abundant grassland orb-weaver spiders (Rodrigues et al. 2009; Nogueira and Pinto-da-Rocha 2016), Argiope argentata (Araneae: Araneidae) and Alpaida quadrilorata (Araneae: Araneidae) (Fig. 1). We hypothesize that the population of both spider species are directly affected by different levels of grazing disturbance. We predict that low and intermediate grazing will benefit both species but they will respond differently from each other. These different responses would be linked to each specific species behavior of colonization and web placement (see Methods below). Hence, vegetation removal will negatively affect their habitat use and abundance, primarily by heavy grazing.
Methods
Species studied
The first species, A. argentata, has a broad range distribution, from Canada to Argentina (Agnarsson et al. 2016; World Spider Catalog 2023) and inhabits low above-ground plants on grassland and margins of roads and trails (Robinson 1969). The second species, A. quadrilorata, is distributed in Argentina, Brazil, Paraguay and Uruguay (Vasconcellos-Neto et al. 2017; World Spider Catalog 2023) and it is known to inhabit, almost exclusively, plants with rosette-shaped leaves that have similar architecture to bromeliads Uruguay (Levi 1988; Vasconcellos-Neto et al. 2017; Hesselberg et al. 2023).
Study site and sampling design
Our study took place in the Pampa grasslands, southern South America. The climate is subtropical with hot, dry summers and humid-cold winters, classified as “Cfa” by the Köppen-Geiger (Kottek et al. 2006). Temperatures vary from a minimum of 7°C surpassing 40°C in summer and from 4°C to 28°C in winter. Rainfall ranges between 1,200 and 1,600 mm throughout the year (Kottek et al. 2006). Sampling occurred at Estação Experimental Agronômica da Universidade Federal do Rio Grande do Sul (UFRGS) located in Eldorado do Sul municipality, Rio Grande do Sul, Brazil (30º06’08’’S; 51º40’56’’W). Since 1987, an experiment called Nativão has been conducted to assess the effects of different intensities of cattle grazing in an area that covers about 52 hectares (Nabinger et al. 2009). In the year 2000, the area was subdivided into 14 plots with different cattle grazing treatments that vary in fixed and daily levels of grass forage supply for cattle, expressed in kg of vegetal dry matter [DM]/100 kg of live weight [LW] (% LW). In this way, these areas are defined by the percentage of vegetal dry matter remaining, meaning the less vegetal dry matter that remains, the greater the grazing pressure (Nabinger et al. 2009).
Six plots were selected for sampling: two plots (3.05 ha and 3.14 ha) with high grazing disturbance (4% LW, around 0.86 Animal Units (AU)/ha/year); two plots (2.73 ha and 3.67 ha) of moderate disturbance (8% LW, around 0.59 AU/ha/year); and two plots (5.27 ha and 5.42 ha) of low grazing disturbance (16% LW, around 0.45 AU/ha/year) (Nabinger et al. 2009). Considering the known home range and movement capacity of one of our target species (Craig et al. 2001), we superimposed a grid on the top of each of the six plots with cell size 5 × 5 m, using the QGIS software (QGIS.org 2020). From the total of cells per plot, a subgroup of 50 cells was randomly sorted for all surveys (50 cells per plot, 300 in total). Then, on each campaign, 16 randomly selected cells were surveyed from the 50 pre-selected cells of each plot (96 in total per campaign). Lastly, the order that the plots were surveyed was always randomized in each campaign.
Seven-monthly campaigns were conducted in year 1, from October 2017 to April 2018, and six-monthly campaigns in year 2, from October 2018 to April 2019, during austral spring and summer, when spiders are more active (Nei et al. 2015). Each campaign was composed of two days (surveys) and species were surveyed in the field from dawn to mid-day (06:00 am to 12:30 pm) and from afternoon to dusk (03:00 pm to 09:00 pm). Cells were surveyed until exhaustion, counting adults and juveniles of A. argentata and A. quadrilorata species. Two to three trained observers were deployed on each campaign (a total of eight people through the experiment).
Data analysis
Environmental variables were registered through the campaigns and surveys to be used as occupancy, abundance, and detection predictor variables. To estimate occupancy probability and abundance, we considered vegetation density and the quadratic effect of vegetation density as spatial variables. Although vegetation density is directly correlated with the different grazing treatments in our field site, during our fieldwork we detected variation in vegetation height within the same plots. Therefore, vegetation density was obtained by taking four photos of the vegetation on each cell and year, using a 1 × 1-m white cardboard as a background to measure vegetation density on every photo. Then, we used ImageJ software (Schneider et al. 2012) to convert images to black and white scale hence, the black pixels were counted as a measure of vegetation density in contrast with the white cardboard background (Ford et al. 2017). The arithmetic mean of black pixels between the four photos was considered a proxy of vegetation density for each cell and in each year. To estimate detection probability, we included air temperature (degrees Celsius) and time (expressed as minutes after midnight) as temporal predictors. Air temperature was measured three times during each survey (beginning, middle and end). Time was taken at the beginning of each cell survey. Moreover, detection probability was also estimated as a function of vegetation density and the quadratic effect of vegetation density. All numeric variables (temperature, time, and vegetation density) were standardized to have zero mean and one standard deviation.
With only two years of sampling, unknown heterogeneity is more likely to be present in our data, hence, fitting a multi-season model would not be the best option (MacKenzie et al. 2002, 2003). Therefore, we fitted single-season models independently for each sampled year and species. We estimated occupancy (Ψ) and detection (p) probabilities using occupancy modeling (MacKenzie et al. 2002). To estimate abundance (N) we fitted N-Mixture models (Royle 2004) using counts of spiders per cell as our response. We used Akaike’s Information Criterion (AIC) to compare and rank occupancy and N-Mixture models considering models with Delta AIC ≤ 2 that are best supported (Arnold 2010) (Supplementary Material 1). Models were model-averaged to provide parameter estimates. Models were built using the ‘unmarked’ package (Chandler et al. 2021), and model-averaged using ‘MuMIn’ package (Bartoń 2019), both in the software R (Team 2022). The detection probability estimates and the effects of temporal variables are not discussed but are presented in the supplementary material (Supplementary Material 1).
Results
A total of 889 individuals of A. argentata (25 in high, 278 in moderate and 586 in low grazing) were found in year one and 883 individuals (83 in high, 510 in moderate and 290 in low grazing) were found in year two. For A. quadrilorata, a total of 430 individuals (one in high, 266 in moderate and 163 in low grazing) were found in year one and 348 individuals (three in high, 263 in moderate and 82 in low grazing) were found in year two.
Argiope argentata occupancy and abundance estimates
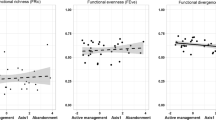
In year one, A. argentata occupancy estimates strongly increased with vegetation density but not significantly (occupancy βVegY1 = 40.404, p = 0.087) while its abundance was negatively affected by the quadratic effect of vegetation density, showing a hump shaped curve (abundance βVeg2Y1 = -0.147, p = 0.050). In this case, A. argentata increase its abundance with the increase of vegetation density (predicted mean abundance of 57.12, a minimum of 21.71 and a maximum of 96.91 individuals) until a point where starts to lose abundance with the highest density we found (Fig. 2 and Supplementary Material 1). In year two, occupancy estimates increased with vegetation density (occupancy βVegY2 = 2.953, p = 0.017) while abundance estimates were negatively affected by the quadratic effect of vegetation density, showing the same hump shaped curve of year one (abundance βVeg2Y2 = -0.343, p = 7.51e-4, predicted mean abundance of 40.55, a minimum of 4.75 and a maximum of 80.20 individuals).
Occupancy (upper) and abundance (lower) estimates for Argiope argentata in year one (left, plot A and C) and year two (right, plot B and D). Red lines indicate mean occupancy estimates for years one and two (plots A and B). Blue lines indicate mean abundance estimates for years one and two (plots C and D). Gray shadows indicate standard deviations
Alpaida quadrilorata occupancy and abundance estimates
In year one, neither occupancy nor abundance estimates of A. quadrilorata were affected by vegetation density (occupancy βVegY1 = -0.192, p = 0.342; and abundance βVegY1 = -0.008, p = 0.946, predicted mean abundance of 44.58, a minimum of 19.91 and a maximum of 106.09 individuals, Fig. 3). In year two, we found the same trend, neither occupancy nor abundance estimates of A. quadrilorata were affected by vegetation density (occupancy βVegY2 = 1.714, p = 0.059; and abundance βVeg2Y2 = 0.132, p = 0.814, predicted mean abundance of 43.07, a minimum of 13.85 and a maximum of 167.45 individuals Fig. 3).
Occupancy and abundance estimates of Alpaida quadrilorata in year one (left, plot A and C) and year two (right, plot B and D). Red lines indicate mean occupancy estimates for years one and two (plots A and B). Blue lines indicate mean abundance estimates for years one and two (plots C and D). Gray shadows indicate standard deviations
Discussion
Based on the results, our findings partially align with our hypothesis that both spider populations are influenced by grazing disturbances, consistent with the broad trend observed in the orb-weaver community. However, only A. argentata significantly responded to vegetation density, corroborating past findings where orb-weaver spiders seem to benefit from intermediate levels of grazing on natural grasslands (Hu et al. 2019; Wang and Tang 2019; Ferreira et al. 2020; Oyarzabal and Guimarães 2021). Moreover, we also predicted correctly that each species would respond differently to grazing, since their behavior is an important feature in this case. Interestingly though, we end up showing that grazing impact can be positive, negative or neutral on spider species, with results being dependent of the spiders group or species (Szmatona-Túri et al. 2017; Samu et al. 2018; Hu et al. 2019; Wang and Tang 2019; Ferreira et al. 2020; da Silva Bomfim et al. 2021; Oyarzabal and Guimarães 2021).
In terms of behavior, both orb-weaver spiders respond differently to grazing pressure, likely due to their specific microhabitat requirements. The species A. argentata can be easily found on the grass, using the leaves, stems and patches to construct their webs (Robinson 1969; Ayoub et al. 2023; Hesselberg et al. 2023), which may reach up to 100 centimeters above ground (Oyarzabal, personal observation). Consequently, the impact of grazing on A. argentata would be direct. As the cattle consume or trample the grass, they inadvertently eliminate potential anchoring points for webs, occasionally even consuming the spiders themselves (Ben-Ari and Inbar 2013; Gish et al. 2017). For A. quadrilorata, though, we found the species exclusively inhabiting Eryngium horridum Malme (referred to as Gravatá or Caraguatá), a plant characterized by rosette-shaped leaves and thorns. This plant is not consumed by cattle and is also resistant to its trampling (Balph and Malecheck 1985; Fidelis et al. 2009; Kurtz et al. 2018; Boavista et al. 2019). Consequently, given this intricate ecological association between the spider, its host plant, and cattle, the grazing effect on A. quadrilorata would be indirect.
It is also important to note the slightly decrease in A. argentata abundance in low grazed areas compared to intermediate grazed areas (Fig. 2). Despite the vegetation on low grazed areas being very dense, harder to walk through and, consequently to find the spiders (Oyarzabal, personal observation), our models showed a higher estimate of detection on low grazed areas (βGrazLowY1 = 3.306, p = < 2e-16) then intermediate grazed areas (βGrazIntY1 = 2.429, p = < 2e-16, see more in the Supplementary Material 1). Since our estimates are already corrected by the imperfect detection (MacKenzie et al. 2002; Guillera-Arroita 2017), it is more likely that, in fact, there is fewer individuals in low grazed areas than in intermediate grazed areas. Therefore, our results are more likely explained by the intermediate disturbance hypothesis, corroborating previous finds for orb-weavers (Oyarzabal and Guimarães 2021), where certain levels of disturbance can promote a higher environmental heterogeneity and hence, enhancing species occupancy and abundance (Willig and Presley 2018; Gao and Carmel 2019; Wang and Tang 2019; Mestre et al. 2020; Oyarzabal and Guimarães 2021).
In the face of disturbance, orb-weavers and other spiders are known to disperse through ballooning (Eberhard 1987; Sheldon et al. 2017; Piacentini et al. 2021). Therefore, dispersal would be a good alternative for both species since the constant grazing could increase the energy cost of rebuilding a web while lowering their feeding capacity (Prestwich 1977; Janetos 1982; Tanaka 1989; Uetz 1992; Fischer et al. 2019). However, concrete evidence for ballooning behavior exists solely for A. argentata, which commonly occur more in juveniles than adults (Bell et al. 2005; Agnarsson et al. 2016), whereas support for A. quadrilorata ballooning is restricted to its genus, Alpaida (Eberhard 1987). The challenge, though, would not be the dispersion but rather finding plant structures for A. argentata and a host plant for A. quadrilorata. In this case, the species would need some chemical, physiological or mechanical mechanism to identify the plants. Spiders have a complex chemical communication system that involves pheromones for mate and offspring recognition (Guimarães et al. 2018; Fischer et al. 2019; Beyer et al. 2021). However, few authors suggest the attractiveness of plant chemicals for spiders (Fischer et al. 2018, 2019, 2021). Moreover, these species have poor vision compared to other spiders like jumping spiders (Salticidae) (Pollard et al. 1987; Richman and Jackson 1992). Hence, they would only perceive light or shade incidence in the environment, which we know is important for hunting strategies (Herberstein et al. 2000; Blamires et al. 2007; da Silva et al. 2021). Even so, the mechanisms of how orb-weaver spiders perceive a suitable habitat to build their webs is still unclear. Hence, delving into light incidence and shade coverage emerges as promising avenues to investigate habitat choice in orb-weaver spiders.
Besides the effects on web displacement, grazing can subsequently affect webs architecture and prey caught ability (Sanders et al. 2015; Hesselberg et al. 2023). This is particularly harmful to orb-weavers since these species cannot hunt without their webs. Furthermore, spiders in general have a great importance in trophic chains as a multi-level generalist predator, being mainly responsible to control other arthropods (Sanders et al. 2015; Ludwig et al. 2018; Yadav and Kumar 2021). Thus, their absence in agroecosystems certainly jeopardize these habitats ecological balance (Dennis et al. 2015; Sanders et al. 2015; Oyarzabal and Guimarães 2021; Hesselberg et al. 2023). Therefore, to avoid cascading effects that goes from the rise of insect pests, to the starvation of vertebrate mesopredators (e.g., birds and reptiles), to the loss of farmers monetary values (Goosey et al. 2019; Goulson 2019; Aguilera et al. 2021; Yadav and Kumar 2021), is of utmost importance to conserve natural grasslands environments, lowering or subsiding the chronic grazing management.
As advocated by other authors (Meadows et al. 2017; Barton et al. 2020) and partially supported by our results, the use of arthropod generalists seems promising as a proxy to study management, chronic anthropogenic disturbances, and conservation. Furthermore, spiders and other arthropods may be suitable candidates to study fine-scale climate change (Staude et al. 2018; Høye 2020). Variations in temperature and rainfall have been affecting arthropod survival, reproduction, body size, clutch size, behavior, and physiology (Supriya et al. 2019; Walsh et al. 2019; Høye 2020), as observed in chordates such as anurans (González-del-Pliego et al. 2020), reptiles (Diele-Viegas et al. 2020), birds (Bateman et al. 2020), and mammals (Mitchell et al. 2018). The conservation and protection of charismatic animal species (e.g., mammals), which may function as umbrella species for the environment (Schlagloth et al. 2018; Wang et al. 2021), is undoubtedly important. However, given the importance of arthropods in food webs, both as predators and prey and in the ecosystem functioning, their disappearance induced by anthropogenic actions may lead to unpredictable ecosystem dynamics, undoubtedly cascading onto ecosystem services and hence jeopardizing landscape conservation (Klaus et al. 2013; Blubaugh et al. 2017; Goulson 2019; Samways et al. 2020).
In conclusion, our study provides valuable insights into the complex interplay between cattle grazing and its impact on orb-weaver spider populations, specifically A. argentata and A. quadrilorata. While our findings partially align with our initial hypothesis that grazing disturbances influence spider populations, they also reveal the intricate nature of these effects. While A. argentata seems to respond directly to grazing, A. quadrilorata, which exclusively inhabits the cattle-resistant Eryngium horridum, is indirectly affected by grazing. Therefore, considering that grazing may not be the best solution for all types of grassland management (Helden et al. 2020), we are careful to say that a moderate grazing, even with a limited animal load, up to 0.6 AU/ha/year (Jansen et al. 2013; Clendenin 2016; Toupet et al. 2020), might preserve spiders and other generalist predators that are intrinsically linked to vegetation structure. Moreover, the ability of these spiders to disperse through ballooning offers a potential survival strategy in the face of grazing pressure, but it presents challenges related to identifying suitable habitats. This highlights promising avenues for future research, particularly regarding how light incidence and shade coverage influence habitat choice for orb-weaver spiders. Besides that, since grazing can also affect web architecture as well as prey’s availability, future researches could also focus on changes in orb-weavers web design and prey caught, based on different grazing levels. In light of these findings, it is imperative that we strive for an optimal balance in managing grazing practices and conserving habitats to ensure the survival of orb-weaver spiders and other arthropods. Recognizing the value of these small but ecologically significant creatures is essential for maintaining the health and stability of our ecosystems and the services they provide (Meadows et al. 2017; Fernández-Tizón et al. 2020).
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
References
Agnarsson I, LeQuier SM, Kuntner M et al (2016) Phylogeography of a good caribbean disperser: Argiope argentata (Araneae, Araneidae) and a new ‘cryptic’ species from Cuba. Zookeys 2016:25–44. https://doi.org/10.3897/zookeys.625.8729
Aguilera G, Riggi L, Miller K et al (2021) Organic fertilisation enhances generalist predators and suppresses aphid growth in the absence of specialist predators. J Appl Ecol 58:1455–1465. https://doi.org/10.1111/1365-2664.13862
Antongiovanni M, Venticinque EM, Matsumoto M, Fonseca CR (2020) Chronic anthropogenic disturbance on Caatinga dry forest fragments. J Appl Ecol 57:2064–2074. https://doi.org/10.1111/1365-2664.13686
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl Manage 74:1175–1178. https://doi.org/10.2193/2009-367
Ayoub NA, DuMez L, Lazo C et al (2023) Orb weaver aggregate glue protein composition as a mechanism for rapid evolution of material properties. Front Ecol Evol 11:1–14. https://doi.org/10.3389/fevo.2023.1099481
Balph DF, Malecheck JC (1985) Cattle trampling of crested wheatgrass under short-duration grazing. J Range Manag 38:226. https://doi.org/10.2307/3898971
Barton BT, Hill JVG, Wolff CL et al (2020) Grasshopper consumption by grey wolves and implications for ecosystems. Ecology 101:1–4. https://doi.org/10.1002/ecy.2892
Bartoń K (2019) Mu-MIn. Multi-model inference
Bateman BL, Wilsey C, Taylor L et al (2020) North American birds require mitigation and adaptation to reduce vulnerability to climate change. Conserv Sci Pract 2:1–18. https://doi.org/10.1111/csp2.242
Bell JR, Bohan DA, Shaw EM, Weyman GS (2005) Ballooning dispersal using silk: world fauna, phylogenies, genetics and models. Bull Entomol Res 95:69–114. https://doi.org/10.1079/ber2004350
Ben-Ari M, Inbar M (2013) When herbivores eat predators: Predatory insects effectively avoid incidental ingestion by mammalian herbivores. PLoS ONE 8. https://doi.org/10.1371/journal.pone.0056748
Beyer M, Mangliers J, Tuni C (2021) Silk-borne chemicals of spider nuptial gifts elicit female gift acceptance. Biol Lett
Blackledge TA, Wenzel JW (1999) Do stabilimenta in orb webs attract prey or defend spiders? Behav Ecol 10:372–376. https://doi.org/10.1093/beheco/10.4.372
Blamires SJ, Thompson MB, Hochuli DF (2007) Habitat selection and web plasticity by the orb spider Argiope keyserlingi (Argiopidae): do they compromise foraging success for predator avoidance? Austral Ecol 32:551–563. https://doi.org/10.1111/j.1442-9993.2007.01727.x
Blubaugh CK, Widick IV, Kaplan I (2017) Does fear beget fear? Risk-mediated habitat selection triggers predator avoidance at lower trophic levels. Oecologia 185:1–11. https://doi.org/10.1007/s00442-017-3909-1
Boavista Lda, Trindade R, Overbeck JPP, Müller GE SC (2019) Effects of grazing regimes on the temporal dynamics of grassland communities. Appl Veg Sci 22:326–335. https://doi.org/10.1111/avsc.12432
Chandler R, Kellner K, Fiske I et al (2021) Package ‘unmarked’ ver. 1.1.1
Clendenin G (2016) Common rangeland plants of west central Texas. Texas A&M University, Texas
Cory AL, Schneider JM (2018) Effects of social information on life history and mating tactics of males in the orb-web spider Argiope bruennichi. Ecol Evol 8:344–355. https://doi.org/10.1002/ece3.3672
Craig C, Wolf SG, Davis JLD et al (2001) Signal polymorphism in the web-decorating spider Argiope argentata is correlated with reduced survivorship and the presence of stingless bees, its primary prey. Evol (N Y) 55:986–993. https://doi.org/10.1554/0014-3820(2001)055
da Silva GO, Ott R (2017) Short-term spider community monitoring after cattle removal in grazed grassland. Iheringia Série Zool 107:1–8. https://doi.org/10.1590/1678-4766e2017033
da Silva FC, Moleta M, Dos Anjos CA et al (2021) Testing traditional hypotheses about prey capture efficiency in orb-web spiders. J Ethol 39:3–8. https://doi.org/10.1007/s10164-020-00663-1
da Silva Bomfim L, Bitencourt JAG, Rodrigues ENL, Podgaiski LR (2021) The role of a rosette-shaped plant (Eryngium Horridum, Apiaceae) on grassland spiders along a grazing intensity gradient. Insect Conserv Divers. https://doi.org/10.1111/icad.12475
Dennis P, Skartveit J, Kunaver A, McCracken DI (2015) The response of spider (Araneae) assemblages to structural heterogeneity and prey abundance in sub-montane vegetation modified by conservation grazing. Glob Ecol Conserv 3:715–728. https://doi.org/10.1016/j.gecco.2015.03.007
Diele-Viegas LM, Figueroa RT, Vilela B, Rocha CFD (2020) Are reptiles toast? A worldwide evaluation of Lepidosauria vulnerability to climate change. Clim Change 159:581–599. https://doi.org/10.1007/s10584-020-02687-5
Eberhard WG (1987) How spiders initiate airborne lines. J Arachnol 15:1–9
Fernández-Tizón M, Emmenegger T, Perner J, Hahn S (2020) Arthropod biomass increase in spring correlates with NDVI in grassland habitat. Sci Nat 107. https://doi.org/10.1007/s00114-020-01698-7
Ferreira PMA, Andrade BO, Podgaiski LR et al (2020) Long-term ecological research in southern Brazil grasslands: effects of grazing exclusion and deferred grazing on plant and arthropod communities. PLoS ONE 15:1–23. https://doi.org/10.1371/journal.pone.0227706
Fidelis A, Overbeck GE, Pillar VD, Pfadenhauer J (2009) The ecological value of Eryngium Horridum in maintaining biodiversity in subtropical grasslands. Austral Ecol 34:558–566. https://doi.org/10.1111/j.1442-9993.2009.01959.x
Filazzola A, Brown C, Dettlaff MA et al (2020) The effects of livestock grazing on biodiversity are multi-trophic: a meta-analysis. Ecol Lett 23:1298–1309. https://doi.org/10.1111/ele.13527
Fischer A, Ayasse M, Andrade MCB (2018) Natural compounds as spider repellents: fact or myth? J Econ Entomol 111:314–318. https://doi.org/10.1093/jee/tox339
Fischer A, Hung E, Gries G (2019) Female false black widow spiders, Steatoda grossa, recognize webs based on physical and chemical cues. Entomol Exp Appl 167:803–810. https://doi.org/10.1111/eea.12825
Fischer A, MacLennan S, Gries R, Gries G (2021) Herbivore-induced plant volatiles do not affect settling decisions by synanthropic spiders. https://doi.org/10.1007/s00049-021-00340-w. Chemoecology
Ford H, Evans B, Van Klink ROEL et al (2017) The importance of canopy complexity in shaping seasonal spider and beetle assemblages in saltmarsh habitats. Ecol Entomol 42:145–155. https://doi.org/10.1111/een.12373
Gagnon K, Gräfnings M, Boström C (2019) Trophic role of the mesopredatory three-spined stickleback in habitats of varying complexity. J Exp Mar Bio Ecol 510:46–53. https://doi.org/10.1016/j.jembe.2018.10.003
Gao J, Carmel Y (2019) Can the intermediate disturbance hypothesis explain grazing-diversity relations at a global scale? Oikos 129:493–502. https://doi.org/10.1111/oik.06338
Gish M, Ben-Ari M, Inbar M (2017) Direct consumptive interactions between mammalian herbivores and plant-dwelling invertebrates: prevalence, significance, and prospectus. Oecologia 183:347–352. https://doi.org/10.1007/s00442-016-3775-2
González-del-Pliego P, Scheffers BR, Freckleton RP et al (2020) Thermal tolerance and the importance of microhabitats for Andean frogs in the context of land use and climate change. J Anim Ecol 89:2451–2460. https://doi.org/10.1111/1365-2656.13309
Goosey HB, Smith JT, O’Neill KM, Naugle DE (2019) Ground-Dwelling Arthropod Community response to Livestock Grazing: implications for Avian Conservation. Environ Entomol 48:856–866. https://doi.org/10.1093/ee/nvz074
Goulson D (2019) The insect apocalypse, and why it matters. Curr Biol 29:R967–R971. https://doi.org/10.1016/j.cub.2019.06.069
Guillera-Arroita G (2017) Modelling of species distributions, range dynamics and communities under imperfect detection: advances, challenges and opportunities. Ecography (Cop) 40:281–295. https://doi.org/10.1111/ecog.02445
Guimarães IDC, Cardoso CAL, Caramão EB et al (2018) Analysis of cuticular chemical profiles of Latrodectus geometricus (Araneae: Theridiidae) females and juveniles using GC×GC/qMS. Ciência e Nat 40:1. https://doi.org/10.5902/2179460x29908
Hashemi A, Aghbash FG, Zarafshar M, Bazot S (2019) 80-years livestock transit impact on permanent path soil in Zagros oak forest, Iran. Appl Soil Ecol 138:189–194. https://doi.org/10.1016/j.apsoil.2019.03.004
Helden AJ, Chipps J, McCormack S, Pereira L (2020) Is grazing always the answer to grassland management for arthropod biodiversity? Lessons from a gravel pit restoration project. J Insect Conserv 24:655–670. https://doi.org/10.1007/s10841-020-00243-1
Herberstein ME, Craig CL, Elgar MA (2000) Foraging strategies and feeding regimes: web and decoration investment in Argiope keyserlingi Karsch (Araneae: Araneidae). Evol Ecol Res 2:69–80
Hesselberg T, Boyd KM, Styrsky JD, Gálvez D (2023) Host plant specificity in web-building spiders. Insects 14:1–20. https://doi.org/10.3390/insects14030229
Høye TT (2020) Arthropods and climate change – arctic challenges and opportunities. Curr Opin Insect Sci 41:40–45. https://doi.org/10.1016/j.cois.2020.06.002
Hu LJ, Wang W, Cheng Y, Guo Y (2019) Effects of grazing livestock on grassland functioning may depend more on grazing intensity than livestock diversity. Proc Natl Acad Sci U S A 116:18762–18763. https://doi.org/10.1073/pnas.1911488116
Janetos AC (1982) Foraging tactics of two guilds of web-spinning spiders. Behav Ecol Sociobiol 10:19–27. https://doi.org/10.1007/BF00296392
Jansen R, Makaka L, Little IANT (2013) Response of ground-dwelling spider assemblages (Arachnida, Araneae) to Montane Grassland management practices in South Africa. https://doi.org/10.1111/icad.12013
Klaus VH, Kleinebecker T, Prati D et al (2013) Agriculture, ecosystems and Environment does organic grassland farming benefit plant and arthropod diversity at the expense of yield and soil fertility ? 177:1–9. https://doi.org/10.1016/j.agee.2013.05.019
Kottek M, Grieser J, Beck C et al (2006) World Map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263. https://doi.org/10.1127/0941-2948/2006/0130
Kurtz DB, Giese M, Asch F et al (2018) Effects of high impact grazing on species diversity and plant functional groups in grasslands of Northern Argentina. https://doi.org/10.3390/su10093153. Sustain 10:
Lesmeister DB, Nielsen CK, Schauber EM, Hellgren EC (2015) Spatial and temporal structure of a Mesocarnivore Guild in Midwestern North America. Wildl Monogr 191:1–61. https://doi.org/10.1002/wmon.1015
Levi HW (1988) The neotropical orb-weaving spiders of the genus Alpaida (Araneae: Araneidae). Bull Museum Comp Zool 151:365–487
Ludwig L, Barbour MA, Guevara J et al (2018) Caught in the web: spider web architecture affects prey specialization and spider–prey stoichiometric relationships. Ecol Evol 8:6449–6462. https://doi.org/10.1002/ece3.4028
Macé OG, Ebeling A, Eisenhauer N et al (2019) Variations in trophic niches of generalist predators with plant community composition as indicated by stable isotopes and fatty acids. Soil Org 91:45–59. https://doi.org/10.25674/so91204
MacKenzie DI, Nichols JD, Lachman GB et al (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
MacKenzie DI, Nichols JD, Hines JE et al (2003) Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 84:2200–2207. https://doi.org/10.1890/02-3090
Meadows AJ, Owen JP, Snyder WE (2017) Keystone nonconsumptive effects within a diverse predator community. Ecol Evol 7:10315–10325. https://doi.org/10.1002/ece3.3392
Mestre F, Pita R, Mira A, Beja P (2020) Species traits, patch turnover and successional dynamics: when does intermediate disturbance favour metapopulation occupancy? BMC Ecol 20:2. https://doi.org/10.1186/s12898-019-0273-5
Michalko R, Pekár S, Entling MH (2019) An updated perspective on spiders as generalist predators in biological control. Oecologia 189:21–36. https://doi.org/10.1007/s00442-018-4313-1
Mitchell D, Snelling EP, Hetem RS et al (2018) Revisiting concepts of thermal physiology: Predicting responses of mammals to climate change. J Anim Ecol 87:956–973. https://doi.org/10.1111/1365-2656.12818
Muvengwi J, Chikorowondo G, Mbiba M, Gandiwa E (2018) Diversity and abundance of macro-invertebrates on abandoned cattle kraals in a semi-arid savanna. Int J Bus Innov Res 17:8477–8489. https://doi.org/10.1002/ece3.4332
Nabinger C, Ferreira ET, Freitas AK et al (2009) Produção animal com base no campo nativo: aplicações de resultados de pesquisa. In: Pillar VDP, Müller SC, Castilhos ZM, de Jacques S AVÁ (eds) Campos Sulinos - conservação e uso sustentável da biodiversidade, 1st edn. MMA, Brasília, pp 175–198
Narimanov N, Kempel A, van Kleunen M, Entling MH (2021) Unexpected sensitivity of the highly invasive spider Mermessus trilobatus to soil disturbance in grasslands. Biol Invasions 23:1–6. https://doi.org/10.1007/s10530-020-02348-9
Nei E, Rodrigues L, Brescovit AD (2015) On the spider genus Thymoites in the Neotropical Region (Araneae, Theridiidae): nine new species, complementary descriptions and new records. 3972:181–207
Neilly H, Jones H, Schwarzkopf L (2020) Ants drive invertebrate community response to cattle grazing. Agric Ecosyst Environ 290:106742. https://doi.org/10.1016/j.agee.2019.106742
Newsome TM, Greenville AC, Cirovic D et al (2017) Top predators constrain mesopredator distributions. Nat Commun 8:1–7. https://doi.org/10.1038/ncomms15469
Nogueira Ado, Pinto-da-Rocha A R (2016) The effects of habitat size and quality on the orb-weaving spider guild (Arachnida: Araneae) in an Atlantic Forest fragmented landscape. J Arachnol 44:36–45
Oyarzabal G, Guimarães M (2021) Friend and foe? The effects of grassland management on global patterns of spider diversity. Ecol Entomol 46:1195–1204. https://doi.org/10.1111/een.13065
Pett BL, Bailey JJ (2019) Ghost-busting: patch occupancy and habitat preferences of Ocyale ghost (Araneae: Lycosidae), a single site endemic in north-western Madagascar. Austral Entomol 58:875–885. https://doi.org/10.1111/aen.12425
Piacentini LN, Grismado CJ, Aisenberg A et al (2021) Massive spider web aggregations in south American grasslands after flooding. Ecol Entomol 46:1333–1341. https://doi.org/10.1111/een.13080
Pollard SD, Macnab AM, Jackson RR (1987) Communication with chemicals: pheromones and spiders. In: Nentwig W (ed) Ecophysiology of spiders, 1st edn. Springer Berlin, Heidelberg, pp 133–141
Prestwich KN (1977) The energetics of web-building in spiders. Comp Biochem Physiol Part Physiol 57:321–326
QGIS.org (2020) QGIS Geographic Information System
Richman DB, Jackson RR (1992) A review of the ethology of jumping spiders (Araneae, Salticidae). Bull Br Arachnol Soc 9:33–37
Rito KF, Arroyo-Rodríguez V, Queiroz RT et al (2017) Precipitation mediates the effect of human disturbance on the Brazilian caatinga vegetation. J Ecol 105:828–838. https://doi.org/10.1111/1365-2745.12712
Robinson MH (1969) Predatory behavior of Argiope argentata (Fabricius). Am Zool 9:161–173
Rodrigues ENL, Mendonça MDS Jr., Ott R (2009) Spider diversity in a rice agroecosystem and adjacent areas in southern Brazil. Rev Colomb Entomol 35:89–97
Royle JA (2004) N-Mixture models for estimating Population size from spatially replicated counts. Biometrics 60:108–115. https://doi.org/10.1111/j.0006-341X.2004.00142.x
Samu F, Horváth A, Neidert D et al (2018) Metacommunities of spiders in grassland habitat fragments of an agricultural landscape. Basic Appl Ecol 31:92–103. https://doi.org/10.1016/j.baae.2018.07.009
Samways MJ, Barton PS, Birkhofer K et al (2020) Solutions for humanity on how to conserve insects. Biol Conserv 242. https://doi.org/10.1016/j.biocon.2020.108427
Sanders D, Vogel E, Knop E (2015) Individual and species-specific traits explain niche size and functional role in spiders as generalist predators. J Anim Ecol 84:134–142. https://doi.org/10.1111/1365-2656.12271
Schlagloth R, Santamaria F, Golding B, Thomson H (2018) Why is it important to use Flagship species in Community Education? The Koala as a case study. Anim Stud J 7:127
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Sheldon KS, Zhao L, Chuang A et al (2017) Revisiting the Physics of Spider Ballooning. In: Association for Women in Mathematics Series. pp 163–178
Staude IR, Vélez-Martin E, Andrade BO et al (2018) Local biodiversity erosion in south Brazilian grasslands under moderate levels of landscape habitat loss. J Appl Ecol 55:1241–1251. https://doi.org/10.1111/1365-2664.13067
Supriya K, Moreau CS, Sam K, Price TD (2019) Analysis of tropical and temperate elevational gradients in arthropod abundance. Front Biogeogr 11:0–11. https://doi.org/10.21425/F5FBG43104
Szmatona-Túri T, Vona-Túri D, Magos G, Urbán L (2017) The effect of grassland management on diversity and composition of ground-dwelling spider assemblages in the Mátra Landscape Protection Area of Hungary. Biol 72:642–651. https://doi.org/10.1515/biolog-2017-0075
Szmatona-Túri T, Vona-Túri D, Urbán L, Magos G (2018) Effect of grazing intensity on diversity of ground-dwelling spiders of grassy and shrubby habitats. Acta Zool Bulg 70:195–202
Tälle M, Deák B, Poschlod P et al (2016) Grazing vs. mowing: a meta-analysis of biodiversity benefits for grassland management. Agric Ecosyst Environ 222:200–212. https://doi.org/10.1016/j.agee.2016.02.008
Tanaka K (1989) Energetic cost of web construction and its effect on web relocation in the web-building spider Agelena limbata. Oecologia 81:459–464. https://doi.org/10.1007/BF00378952
Team RC (2022) R: a Language and Environment for Statistical Computing. R found. Stat. Comput 1
Torma A, Császár P, Bozsó M et al (2019) Species and functional diversity of arthropod assemblages (Araneae, Carabidae, Heteroptera and Orthoptera) in grazed and mown salt grasslands. Agric Ecosyst Environ 273:70–79. https://doi.org/10.1016/j.agee.2018.12.004
Toupet R, Gibbons AT, Goodacre SL, Bell MJ (2020) Effect of herbage density, height and age on nutrient and invertebrate generalist predator abundance in permanent and temporary pastures. Land 9:1–13. https://doi.org/10.3390/LAND9050164
Uetz GW (1992) Foraging strategies of spiders. Trends Ecol Evol 7:155–159. https://doi.org/10.1016/0169-5347(92)90209-T
Vasconcellos-Neto J, Messas YF, Souza HS et al (2017) Spider–plant interactions: an Ecological Approach. In: Viera C, Gonzaga MO (eds) Behaviour and Ecology of spiders. Contributions from the Neotropical Region, 1st edn. Springer Nature, Cham, Switzerland, pp 165–214
Walsh BS, Parratt SR, Hoffmann AA et al (2019) The impact of Climate Change on Fertility. Trends Ecol Evol 34:249–259. https://doi.org/10.1016/j.tree.2018.12.002
Wang C, Tang Y (2019) A global meta-analyses of the response of multi-taxa diversity to grazing intensity in grasslands. Environ Res Lett 14:114003. https://doi.org/10.1088/1748-9326/ab4932
Wang Y, Allen ML, Wilmers CC (2015) Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biol Conserv 190:23–33. https://doi.org/10.1016/j.biocon.2015.05.007
Wang F, Winkler J, Viña A et al (2021) The hidden risk of using umbrella species as conservation surrogates: a spatio-temporal approach. Biol Conserv 253. https://doi.org/10.1016/j.biocon.2020.108913
Weiss K, Schneider JM (2021) Family-specific chemical profiles provide potential kin recognition cues in the sexually cannibalistic spider Argiope bruennichi. Biol Lett 17. https://doi.org/10.1098/rsbl.2021.0260
Whitney TD, Sitvarin MI, Roualdes EA et al (2018) Selectivity underlies the dissociation between seasonal prey availability and prey consumption in a generalist predator. Mol Ecol 27:1739–1748. https://doi.org/10.1111/mec.14554
Willig MR, Presley SJ (2018) Biodiversity and Disturbance. Elsevier Inc.
Wimp GM, Ries L, Lewis D, Murphy SM (2019) Habitat edge responses of generalist predators are predicted by prey and structural resources. Ecology 100:1–10. https://doi.org/10.1002/ecy.2662
World Spider Catalog (2023) World Spider Catalog. Version 24.5. In: Nat. Hist. Museum Bern. https://wsc.nmbe.ch/. Accessed 14 Aug 2023
Wray AK, Peery MZ, Jusino MA et al (2021) Predator preferences shape the diets of arthropodivorous bats more than quantitative local prey abundance. Mol Ecol 30:855–873. https://doi.org/10.1111/mec.15769
Yadav S, Kumar V (2021) Study of a prey-predator model with preventing crop pest using natural enemies and control. AIP Conf Proc 2336. https://doi.org/10.1063/5.0045745
Acknowledgements
We are grateful for CAPES and the scholarship granted to the first author (process #88882.439370/2019-01). We are also grateful for Neotropical Grassland Conservancy and the Student Grant granted in 2018 to the first author. We are thankful for all the people who helped us during fieldwork, mainly Andressa Gigante. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This work was supported by CAPES with a scholarship granted to the first author (process #88882.439370/2019-01) and is part of his PhD degree dissertation (PPGBAN/UFRGS). This work is also supported by the Student Grant provided by the Neotropical Grassland Conservancy in 2018.
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by G.O. The first draft of the manuscript was written by G.O. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oyarzabal, G., Guimarães, M. Strands of connection: unraveling livestock grazing effects on orb-weaver spiders. J Insect Conserv 28, 459–468 (2024). https://doi.org/10.1007/s10841-024-00560-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-024-00560-9