Abstract
Introduction
Knowledge on the mobility of threatened species is a clue to understanding population dynamics and is needed to develop appropriate conservation strategies. Here, we investigate movement patterns of the Bei-Bienko’s Plump Bush-cricket (Isophya beybienkoi), an example of a flightless and critically endangered species endemic to the Slovak Karst (southern Slovakia, Central Europe). The capture-mark-recapture method was used to estimate the mobility of the species using fluorescent dye as a marking medium. We found that the mean (± SD) daily distance travelled by this species was only 3.2 ± 2.6 m, with significant differences between males (4.1 ± 3.0 m) and females (2.7 ± 2.1 m). Our results indicate that I. beybienkoi is a short-distance disperser. Males disappeared faster than females from the study plots (at maximum, two females were recaptured even after 41 days). The observed movement patterns suggest that the most urgent conservation measure for this species is to improve the habitat quality of sites, which suffer from overgrowth, and to maintain the quality of other suitable sites that might increase the size of the existing subpopulations.
Implications for insect conservation
Our results show that I. beybienkoi is a short-distance disperser and wanders only within its optimal habitat. Hence, to incorporate movement behaviour into conservation, one of the measures that should mitigate this threat is to preserve or improve the quality of habitats that suffer from overgrowth, in order to increase the size of existing subpopulations. The observed movement patterns suggest that the species is probably incapable of responding to changes in the availability of suitable habitats by dispersing, indicating a limited exchange of individuals between isolated populations. Thus, to enhance structurally diverse mosaic of high-quality habitats, restoration of migration corridors former used as movement corridors for grazing animals may support the dispersal of the threatened bush-cricket.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Information about animal movement and dispersal have become crucial subjects in conservation biology (Bönsel and Sonneck 2011; Weyer et al. 2012; Kral-O’Brien and Harmon 2021). Knowledge on the mobility of threatened species is a key to understanding population dynamics and is often needed to develop appropriate conservation strategies (McCartney et al. 2006; Rada et al. 2015). The movement patterns of insects are a well-studied concern, especially in orthopteran species (Bönsel and Sonneck 2011; Weyer et al. 2012), because many of them are flightless; thus, they are easy to catch and mark, representing excellent subjects for detailed dispersal studies (Kindvall 1999; Diekötter et al. 2005; Heller 2020). This is especially true in the case of isolated or non-flying species, which are often strongly restricted to their specific habitat (Holuša et al. 2013; Nuhlíčková et al. 2021a, b) and thus unable to cross larger distances, for example through unsuitable habitat. As a result, isolated and small populations that live for a long time in unchanged conditions could evolve a low dispersal ability (Bönsel and Sonneck 2011; Bonte et al. 2012; Cote et al. 2017; Fletcher et al. 2018).
Among bush-crickets (Tettigoniidae), the majority of species show a female-biased sexual sized dimorphism often linked to higher survival rate or longer life spans in the larger sex (Hochkirch et al. 2007; Hochkirch and Gröning 2008). On the other hand, males typically benefit more from rapid development and protandry, as they have to invest more in reproduction (e.g. energetically expensive production of nuptial gifts or calling songs) (Whitman 2008; Lehmann 2012; Sielezniew et al. 2020). Therefore, more conspicuous behaviours, such as greater mobility or dispersal, are often more characteristic for males when searching for females (Ingrisch and Köhler 1998; Wedell and Ritchie 2004; Voigt et al. 2005; Lehmann 2012).
Bei-Bienko’s Plump Bush-cricket (Isophya beybienkoi Mařan 1958) is an example of a flightless and critically endangered species which is endemic to the Slovak Karst (southern Slovakia, Central Europe) (Chobanov et al. 2016). Only limited information exists for this species, except for several studies on its morphology, distribution or bioacoustics (Mařan 1958; Orci et al. 2001; Heller et al. 2004; Krištín et al. 2009, 2020). No information is known about its movement behaviour. Thus, to fill this information gap, a capture-mark-recapture experiment (CMR) was conducted to estimate its mobility (daily distance, movement trajectory, dispersal) and survival. For this purpose, fluorescent dye was used as the marking medium, as it is non-toxic, easy to use and highly visible (Faiman et al. 2021) and therefore suitable for animals with nocturnal activity. Based on previous studies on flightless Orthoptera species (Holuša et al. 2013; Breuer et al. 2017; Heller 2020; Kenyeres and Varga 2023), we expected the movement capability of I. beybienkoi to be low. Similar to other species of the genus Isophya, we also assumed that it would be strongly restricted to its specific habitat, providing individuals with a required food source or shelters in vegetation (Bauer and Kenyeres 2006; Nuhlíčková et al. 2021b). Next, we also expected that males may disappear faster than females in the experiment due to more rapid development (protandry) and higher investments in reproduction (Lehmann 2012; Sielezniew et al. 2020). Finally, our study provides basic information that will be implemented in the conservation strategy of this highly endangered species.
Material and methods
Study species
The genus Isophya comprises about 90 species distributed around the Palearctic (Chobanov 2009). Bei-Bienko’s Plump Bush-cricket occurs in only very small (up to 30 adults/ha) and isolated subpopulations with an area of occurrence of only 24 km2 (Chobanov et al. 2016; Krištín et al. 2020). It is a relatively large (up to 26 mm) species with well-developed acoustic communication (Orci et al. 2001), reproducing from June to September (Chobanov et al. 2016, own unpublished observations). Like other species of this genus, it is characterised by mostly crepuscular to nocturnal activity and a high frequency of male calling song (Berg et al. 1996; Vadkerti and Szövényi 2005). Its occurrence is linked to karstic grasslands up to 800 m a.s.l. (Chobanov et al. 2016).
Study site
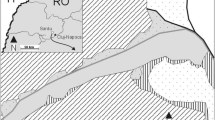
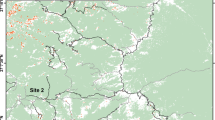
The study was conducted in the Slovak Karst National Park (NP). The karst phenomena is associated with rich xerophilous steppe vegetation on sunny slopes, rocky fields and the edges of plateaux (Dúbravková-Michálková et al. 2008). To obtain data on the species' current distribution, all historical and suitable sites were intensively surveyed prior to this experiment by walking slowly through the relevant habitats and sweep-netting the vegetation (Wagner 2015; Nuhlíčková et al. 2021b). In doing this, a total of 484 km of transects were conducted in suitable habitats in 2021 (254 km) and 2022 (230 km). Based on this survey, two sites (ES) were selected according to the population density and suitability for such a field experiment: (1) Rakaťa meadows (ES1; Fig. 1a): Silická planina plateau, NE of the Silica village, 565 m a.s.l.; slope: 5° and (2) the karstic grasslands of Malý vrch Hill (ES2; Fig. 1b): Silická planina plateau, NE of the Gombasek village, 550 m a.s.l.; slope: 11°. Both sites (ES1 and ES2) were characterised by fringe vegetation (Bromion erecti, Geranion sanguinei) rich in broad-leaved flowering plants in transitional zones between forests edges, xerophilous grasslands or solitary old oaks (Quercus sp.), hornbeams (Carpinus betulus) and meadows (Dúbravková-Michálková et al. 2008; own unpublished data) (Fig. 1). The vegetation of the ES1 was very irregularly grazed by cattle. The territory of ES2 is currently without any management. Both study plots are isolated from other suitable habitats by forests and other habitats unsuitable for this species (see Fig. 3).
Marking procedure
For fluorescence marking of bush-crickets, a non-toxic, water-based SmartWater® solution was used (Faiman et al. 2021), rendering the marked animal visible under UV light (365 nm) (Fig. 2a and b). Each individual (79 males and 113 females) was labelled with a unique code on the pronotum using a permanent non-toxic paint marker. The animal was subsequently painted with light-green fluorescence colour using a thin brush on the abdomen (every side around) and both femurs to maximise the detectability in the field, avoiding any sensitive parts, such as the head, mandibles, eyes or tympanal organ on the front legs (see Fig. 2b). After the marking was done, the coloured individuals were carefully returned to the place of their collection. Sex, GPS location (Garmin GPSMAP 65 EUROPE with Multi-GNSS receiver, with location accuracy about ± 2 m) and time of capture and recapture were recorded before release.
Tracking of bush-cricket movements
The capture session started on 21 June 2022 at ES1 and 26 June 2022 at ES2 (see Online Resource 1), by slow walking and sweep-netting, following line transects arranged by GPS devices. In total, territories covering an area of 20,000 m2 (Fig. 3a) and 22,500 m2 (Fig. 3b) were investigated at ES1 and ES2, respectively, without any re-passing of already inspected vegetation or missing of unchecked habitats.
Recapture controls were performed during night hours (12 h after dusk, usually from 9:30 p.m. to 01:00 a.m.) (Online Resource 1 and Online Resource 2) using a UV flash-torch (UVB-V3-365, USA) and supported by a bat-detector to enhance the search for calling males. In this way, vegetation was carefully examined in the same way as during the marking procedure, following the line transect arranged by GPS devices. For each labelled individual resighted by UV, the new GPS location, date and time were recorded. During the night controls, newly captured individuals that had not been previously labelled were marked and released. The CMR experiment ran for 18 days, until 4 August 2022 (10 controls at ES1, 8 controls at ES2, Online Resource 1). Each control was carried out during calm weather (no wind, clear sky, without rain) to maximise the detection of animals. Labelled individuals that were not recaptured at all during the experiment were assumed to have been consumed by predators or emigrated from the site. For a detailed overview of all captured, marked and recaptured individuals found in this study, see Online Resource 2.
Data analysis
To evaluate the movement pattern of the species, the following linear movement parameters of individuals were calculated: i) the daily distance (average net displacement distance; m/day) representing the straight-line distance covered by an individual within 24 h; ii) the direction of turning angles (°) representing the change of movement direction between consecutive relocations of an individual; iii) movement trajectory (m) – linear distance between consecutive relocations of marked and recaptured individuals; iv) dispersal distance (m) – linear distance between a site where an individual was marked for the first time and recaptured for the last time (i.e. distance of the first and the last location). Movement trajectories were calculated from GPS data using the function as.ltraj in the package “adehabitatLT” (Calenge et al. 2023) of the R 4.3.0 statistical environment (R Core Team 2023). Association between sex and capture/recapture events were evaluated using the Chi-squared test (Pearson’s Chi-squared test with Yates’ continuity correction) in the base R package “stats” (R Core Team 2023). To compare the temporal pattern of recaptures between the sexes, the difference between survival curves from a Kaplan–Meier formula using the functions survfit and surfdiff from the R package “survival” (Therneau and Grambsch 2000; Therneau 2022) was used. We set the day when an individual was marked as day zero.
Generalised linear models (GLMs) with Gamma distribution and identity link were fitted to assess the association between movement trajectory and dispersal as response variables, and time and sex of the crickets as explanatory variables. The number of fixes was included in the models as an offset term. The function glm from the base R package “stats” and subsequently the function Anova (type 3) from the R package “car” (Fox and Weisberg 2019) were used to compute an analysis-of-variance table for the objects of the models. For the GLMs, Nagelkerke’s R2 was computed using the R package “sjstats” (Lüdecke 2022). The function model_check from the R package “performance” (Lüdecke et al. 2021) was used to check the models’ assumptions. To analyse directionality or randomness of the crickets’ movements (i.e. direction of the turning angles), the Rayleigh test of uniformity was used separately for both sexes. To test for differences between sexes in the direction of movement, Watson’s two sample test of homogeneity was used. Both tests are implemented in the R package “circular” (Agostinelli and Lund 2022). Finally, the R packages “ggplot2” (Wickham 2016) and “ggfortify” (Horikoshi and Tang 2016; Tang et al. 2016) were used for plotting.
Results
Altogether, 192 bush-crickets were marked at both study sites (41.1% males and 58.9% females). A total of 44 males and 71 females were recaptured 69 and 150 times, respectively. The overall recapture rate (i.e. the proportion of individuals recaptured at least once) was 59.9%, with fewer males (38.3%) than females (61.7%) recaptured. We found that a lower proportion of unique males were recaptured than marked and released, but the differences between the sexes were not statistically significant (χ2 = 0.14, P = 0.710). However, the proportion of male recaptures was significantly lower than that of females (χ2 = 3.72, P = 0.054), i.e. more unique female individuals were recaptured repeatedly than males. This is due to the faster disappearance of males during recaptures: females were recaptured almost twice as long as males (χ218 = 146.0, P < 0.001; Fig. 4).
The mean daily distance of the bush-crickets was 3.2 m. The mean daily distance of males was higher than that of females (χ2 = 8.93, P = 0.003, R2 = 0.10). Males moved an average of 4.1 m per day, while females 2.7 m per day (Table 1). The direction of turning angles between successive movements was random in both males (Rayleigh test, test statistic = 0.2082, P = 0.287) and females (test statistic = 0.1372, P = 0.226) and no difference was found between the sexes (Watson’s two-sample test, test statistic = 0.06, P > 0.10). The movement trajectory (m) of recaptured individuals was positively associated with observation time (tracking time) (χ2 = 66.64, P < 0.001, R2 = 0.58; Fig. 5a). We found that the bush-crickets moved an average dispersal distance of 16.2 m (Table 1). The dispersal distance (Fig. 5b) was also positively related to time (χ2 = 26.67, P < 0.001), but the strength of this association was weaker (R2 = 0.26) than for the movement trajectory (Fig. 5a and b). Sex did not affect the variability of the response variable, either alone or in interaction with time (χ2 ≤ 1.83, P ≥ 0.18). The longest observed individuals were two females that were recaptured for more than a month (41 days in total), i.e. throughout the entire experiment (Fig. 4). The maximum trajectory travelled by a bush-cricket was 182.7 m (Table 1).
Discussion
Tracking of bush-cricket movements
In this study, we found a very low movement ability of I. beybienkoi, with a mean daily dispersal distance of 3.2 m and a maximum of 13.8 m per day. The main types of movements found in this species were climbing or clambering. Even when disturbed, the bush-cricket preferred falling or slow hiding into the lower vegetation layers rather than jumping. In most of the measured characteristics, movement patterns were similar to other flightless bush-crickets. For example, the predatory katydid Saga pedo moved extraordinary low distances between 0.5 and 2 m in 24 h, but one individual was able to travel up to 330 m in one night (Holuša et al. 2013). In the related Isophya kraussii, Köhler et al. (2019) found that the daily movement (average of 58 days) in males was 6.5 (1.5–9) m and in females only 3.5 (2.0–2.5) m. On the other hand, the large and flightless Mormon cricket (Anabrus simplex) has shown a great variability in daily distances. The animal was found to have travelled from 0.66 m up to 331 m per day (Lorch et al. 2005). Movements in the Cook Strait Giant Weta (Deinacrida rugosa), resident on Matiu-Somes Island, has shown that the average distance between consecutive daytime refuges ranged from 7 to 41 m (Watts et al. 2012). Extremely low daily movements were also detected in two species of endemic grasshoppers from South Africa (Betiscoides meridionalis and B. parva). Approximately 60% of females of both species travelled less than 0.5 m per day, while males of both species crossed cumulative distances up to 15 m during 9 and 10 days (Matenaar et al. 2014). In fact, low movement ability and dispersal are reason why the majority of flightless Orthoptera are known to be at higher extinction risk than well-flying species (Van Dyck and Baguette 2005; Matenaar et al. 2014; Cote et al. 2017). We showed that both sexes of I. beybienkoi featured very low dispersal (on average 16.2 m), with only random directions between successive moves, suggesting routine movements (Van Dyck and Baguette 2005). In this regard, many flightless species limit their movement only to their specific habitat and are therefore unable to cover longer distances, e.g. through unsuitable vegetation (Weyer et al. 2012; Nuhlíčková et al. 2017, 2021b). As a result, even isolation represents a serious threat, especially in species with reduced mobility. For instance, distribution of the related Isophya costata is restricted to small and isolated subpopulations linked to historical refugia (Nuhlíčková et al. 2017, 2021b). The flightless Chorthippus montanus is strongly restricted to its optimal habitat and is probably not able to cross larger distances through unsuitable vegetation (Weyer et al. 2012). Finally, the occurrence of I. beybienkoi is restricted to only small and isolated subpopulations in semi-natural calcareous grasslands, which are threatened by inadequate management leading to succession of its habitat (Chobanov et al. 2016). Thus, the bush-crickets seem to restrict their movements to the routine type – being mostly reflected within its habitat and hence, being sedentary (Van Dyck and Baguette 2005). As a result, species that live in habitats where the conditions remain largely unchanged over the long-term could evolve low dispersal patterns (Van Dyck and Baguette 2005; Bönsel and Sonneck 2011; Bonte et al. 2012; Cote et al. 2017; Fletcher et al. 2018; Bartlow and Agosta 2021).
Recapture rates, survival and sex-specific differences in mobility
At both experimental sites, the recapture rates of bush-crickets decreased over time. This pattern is characteristic, especially for CMR methods, and can generally be attributed to higher mortality of individuals (Morton 1982; Narisu et al. 1999; Hein et al. 2003; Diekötter et al. 2005, 2007). Given the fact that both study plots were isolated from other suitable habitats by forests and other habitats unsuitable for this species, we assume that individuals of I. beybienkoi could not emigrate out of the range of the CMR-area. However, lower recaptures rates of males compared to females may indicate the disappearance of males during the recapture sessions, indicating sex-biased survival caused by specific differences in the behaviour of both sexes, as males mature earlier than females (protandry) and have to invest more in reproduction (Whitman 2008; Lehmann 2012; Sielezniew et al. 2020). For instance, males of Poecilimon thessalicus which were more preferred by conspecific females faced a higher predation risk by the parasitoid fly Therobia leonidei (Lehmann et al. 2001). Spermatophylax of I. kraussii can supply a female’s energy demands for one or two days. As females can mate every two or three days, they may obtain most of their food by mating (Voigt et al. 2005). Females of Gryllus integer from two populations differing in predation pressure did not differ in hiding times compared to males. Males from a high-predation habitat were hidden for a longer time than males from a low-predation habitat (Hedrick and Kortet 2006). Thus, it appears that males have to move towards females, because they spend more time searching for a mate (Wedell and Ritchie 2004; Voigt et al. 2005; Hochkirch et al. 2007; Lehmann 2012). In contrast, females are the sex that benefits from a larger body, possible longer life spans or higher survival rates (Hochkirch et al. 2007; Hochkirch and Gröning 2008; Lehmann 2012). I. beybienkoi females were recaptured at almost twice as long-time intervals than males, and males travelled significantly longer daily distances than females. The longest observed individuals were two females that were recaptured after 41 days, which was longer than known in other (flightless) bush-cricket species (e.g. Heller and von Helversen 1990; Köhler et al. 2019).
The effectiveness of CMR experiments is generally reflected in the recapture rate, which depends on the target species, habitat structure, research intensity and on the way in which individuals are marked (Ingrisch and Köhler 1998). We have shown that the fluorescent dye has very good retention, indicating a suitable product for marking threatened species. However, we were unable to clearly assess whether the lower recapture rates of males were a result of predation or other life history strategies, as indicated by the results above. Therefore, it is possible that the earlier development of I. beybienkoi males (Whitman 2008; Lehmann 2012; Sielezniew et al. 2020) is responsible for the lower recapture rates in later stages of the vegetation season. Hence, further research will be needed to explore in more detail the sex-specific differences between males and females. Furthermore, it is also possible that this species is more mobile than we thought. For example, different mobility of individuals can be affected by stressful conditions (e.g. high population density) or disturbances. While some species show higher dispersal (e.g. Metrioptera roeselii – due to macropterous individuals), other species are only capable of limited mobility (e.g. M. brachyptera) (Poniatowski and Fartmann 2011a, b). However, the dispersal ability of I. beybienkoi was very low, which indicates only a limited exchange of individuals between isolated populations. Based on our results, the bush-cricket is probably unable to respond to changes in the availability of suitable habitats by dispersing and hence may be especially dependent on habitat management activities that promote the long-term stability of existing habitat patches (see Poniatowski and Fartmann 2011b). On the other hand, we found that the majority of recaptured individuals were sitting in a very similar location, usually at the top of a host plant. Thus, our bush-cricket may search for food or mate but returns to a similar place, e.g. a host plant. Such homing behaviour is characteristic for a few nocturnal bush-crickets and katydids (Tettigoniidae), which return, e.g., to their day-time roosts after nightly excursions (Hale and Bailey 2004; Heller 2020). However, the methodological limitations of this study do not yet allow safe conclusions.
Finally, it should be noted that the results obtained in this study are based on the location of individuals using a GPS device with an accuracy of ~ 2 m, which may bias to some extent the results of the subtle movements detected in this endemic bush-cricket. In a fine scale movement tracking study, statistically significant measurement errors of the GPS approach were observed for distances and bearings (Růžičková and Elek 2021). Distances measured by GPS (with an accuracy of ~ 3 m) were approx. 20–30% greater than direct distance-bearing measures. The shorter the distance covered, the greater the relative error, and the average bearing error of GPS was evenly distributed over all directions. These errors were larger under a dense forest canopy (Růžičková and Elek 2021). The implications of these findings are case specific and depend on the objective of the study. In the case of overestimation of movement distances by GPS, our conclusion about the low dispersal capability of I. beybienkoi and related conclusions for conservation management activities that promote the long-term stability of existing habitat patches (see below) remain valid.
In conclusion, our results show that I. beybienkoi is a short-distance disperser and wanders only within its optimal habitat. Hence, to incorporate movement behaviour into conservation, one of the measures that should mitigate this threat is to preserve or improve the quality of habitats that suffer from overgrowth, in order to increase the size of existing subpopulations. The observed movement patterns suggest that the species is probably incapable of responding to changes in the availability of suitable habitats by dispersing, indicating a limited exchange of individuals between isolated populations. Thus, to enhance structurally diverse mosaic of high-quality habitats, restoration of migration corridors former used as movement corridors for grazing animals may support the dispersal of the threatened bush-cricket.
Availability of data and material (data transparency)
Not applicable.
Code availability (software application or custom code)
Not applicable.
Change history
28 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10841-023-00542-3
References
Agostinelli C, Lund U (2022) R package “circular”: Circular Statistics (version 0.4–95). https://r-forge.r-project.org/projects/circular/
Bartlow AW, Agosta SJ (2021) Phoresy in animals: review and synthesis of a common but understudied mode of dispersal. Biol Rev 96:223–246. https://doi.org/10.1111/brv.12654
Bauer N, Kenyeres Z (2006) Habitat preference studies of some species of the genus Isophya Brunner von Wattenwyl, 1878 (Orthoptera: Phaneropteridae) in the western part of the Carpathian Basin. J Orthoptera Res 15:175–185. https://doi.org/10.1665/1082-6467(2006)15[175:hpsoss]2.0.co;2
Berg H, Bieringer G, Sauberer N, Zuna-kratky T (1996) Verbreitung und Ökologie der Grossen Plumpschrecke (Isophya costata) an ihrem westlichen Arealrand (Österreich). Articulata 11:33–45
Bönsel AB, Sonneck AG (2011) Habitat use and dispersal characteristic by Stethophyma grossum: The role of habitat isolation and stable habitat conditions towards low dispersal. J Insect Conserv 15:455–463. https://doi.org/10.1007/s10841-010-9320-4
Bonte D, Van Dyck H, Bullock JM et al (2012) Costs of dispersal. Biol Rev 87:290–312. https://doi.org/10.1111/j.1469-185X.2011.00201.x
Breuer M, Simons IH, Gerlach T, Reinhardt K (2017) Die Größe und Dichte einer population der Plumpschrecke (Isophya kraussii) in der bayerischen Rhön. Articulata 32:125–133
Calenge C, Dray S, Royer M (2023) _adehabitatLT: Analysis of Animal Movements_. R Packag. version 0.3.27. https//cran.r-project.org/package=adehabitatLT
Chobanov DP (2009) Phylogeny and systematics of the Isophya modesta group (Phaneropteridae). Metaleptea 29:20–27
Chobanov DP, Hochkirch A, Iorgu I Ștefan, et al (2016) Isophya beybienkoi. In: IUCN Red List Threat. Species. https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T62147256A62147267.en
Cote J, Bestion E, Jacob S et al (2017) Evolution of dispersal strategies and dispersal syndromes in fragmented landscapes. Ecography (cop) 40:56–73. https://doi.org/10.1111/ecog.02538
Diekötter T, Csencsics D, Rothenbühler C et al (2005) Movement and dispersal patterns in the bush cricket Pholidoptera griseoaptera: The role of developmental stage and sex. Ecol Entomol 30:419–427. https://doi.org/10.1111/j.0307-6946.2005.00714.x
Diekötter T, Speelmans M, Dusoulier F et al (2007) Effects of landscape structure on movement patterns of the flightless bush cricket Pholidoptera griseoaptera. Environ Entomol 36:90–98. https://doi.org/10.1603/0046-225X(2007)36[90:EOLSOM]2.0.CO;2
Dúbravková-Michálková D, Janišová M, Kolbek J et al (2008) Dry grasslands in the Slovensk Kras Mts (Slovakia) and the Aggteleki-Karszt Mts (Hungary) - a comparison of two classification approaches. Hacquetia 7:123–140. https://doi.org/10.2478/v10028-008-0007-2
Faiman R, Krajacich BJ, Graber L et al (2021) A novel fluorescence and DNA combination for versatile, long-term marking of mosquitoes. Methods Ecol Evol 12:1008–1016. https://doi.org/10.1111/2041-210X.13592
Fletcher RJ, Reichert BE, Holmes K (2018) The negative effects of habitat fragmentation operate at the scale of dispersal. Ecology 99:2176–2186. https://doi.org/10.1002/ecy.2467
Fox J, Weisberg S (2019) _An R Companion to Applied Regression_, Third edit. Sage, Thousand Oaks CA
Hale RJ, Bailey WJ (2004) Homing behaviour of juvenile Australian raspy crickets (Orthoptera: Gryllacrididae). Physiol Entomol 29:426–435. https://doi.org/10.1111/j.0307-6962.2004.00412.x
Hedrick AV, Kortet R (2006) Hiding behaviour in two cricket populations that differ in predation pressure. Anim Behav 72:1111–1118. https://doi.org/10.1016/j.anbehav.2006.03.018
Hein S, Gombert J, Hovestadt T, Poethke HJ (2003) Movement patterns of the bush cricket Platycleis albopunctata in different types of habitat: Matrix is not always matrix. Ecol Entomol 28:432–438. https://doi.org/10.1046/j.1365-2311.2003.00531.x
Heller K-G (2020) Diel movement pattern and microhabitat choice in males and females of a flightless phytophagous bush-cricket (Orthoptera: Tettigonioidea: Poecilimon veluchianus). Articulata 35:37–46
Heller KG, von Helversen O (1990) Survival of a Phaneropterid Bush-cricket studied by a new marking technique. Entomol Gen 15:203–208
Heller K-G, Orci KM, Grein G, Ingrisch S (2004) The Isophya species of Central and Western Europe (Orthoptera: Tettigonioidea: Phaneropteridae). Tijdschr Voor Entomol 147:237–258. https://doi.org/10.1163/22119434-900000153
Hochkirch A, Gröning J (2008) Sexual size dimorphism in Orthoptera ( sens. str.) — a review. J Orthoptera Res 17:189–196. https://doi.org/10.1665/1082-6467-17.2.189
Hochkirch A, Gröning J, Krause S (2007) Intersexual niche segregation in Cepero’s Ground-hopper, Tetrix ceperoi. Evol Ecol 21:727–738. https://doi.org/10.1007/s10682-006-9147-3
Holuša J, Kočárek P, Vlk R (2013) Monitoring and conservation of Saga pedo (Orthoptera: Tettigoniidae) in an isolated nothwestern population. J Insect Conserv 17:663–669. https://doi.org/10.1007/s10841-013-9550-3
Horikoshi M, Tang Y (2016) ggfortify: data visualization tools for statistical analysis results. https://cran.r-project.org/package=ggfortify
Ingrisch S, Köhler G (1998) Die Heuschrecken Mitteleuropas. Die neue Brehm-Bücherei, Westarp Wissenschaften, Magdeburg
Kenyeres Z, Varga S (2023) Effects of mowing on Isophya costata, Natura 2000 species (Orthoptera), by direct mortality and management history. J Insect Conserv. https://doi.org/10.1007/s10841-023-00456-0
Kindvall O (1999) Dispersal in a metapopulation of the bush cricket, Metrioptera bicolor (Orthoptera: Tettigoniidae). J Anim Ecol 68q:172–185
Köhler G, Ebeling A, Schumacher J (2019) Untersuchungen an einer Wildpopulation adulter Isophya kraussii Brunner von Wattenwyl, 1878 bei Jena/Thüringen (Orthoptera: Phaneropteridae). Articulata 34:57–70
Kral-O’Brien KC, Harmon JP (2021) The expanding role of movement behavior in insect conservation ecology. Curr Opin Insect Sci 45:69–74. https://doi.org/10.1016/j.cois.2021.02.006
Krištín A, Fabriciusová V, Hrúz V, Kaňuch P (2009) Grashoppers and crickets (Orthoptera) of the National Park Slovenský Kras Karst (E Slovakia). Nat Carp 49:23–32
Krištín A, Jarčuška B, Kaňuch P (2020) An annotated checklist of crickets, grasshoppers and their allies (Orthoptera) in Slovakia. Zootaxa 4869:207–241. https://doi.org/10.11646/zootaxa.4869.2.3
Lehmann GUC (2012) Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettigoniidae), with an emphasis on nuptial gifts, protandry and mate density. Front Zool. https://doi.org/10.1186/1742-9994-9-19
Lehmann GUC, Heller KG, Lehmann AW (2001) Male bushcrickets favoured by parasitoid flies when acoustically more attractive for conspecific females (Orthoptera: Phanopteridae / Diptera: Tachinidae). Entomol Gener 25:135–140
Lorch PD, Sword GA, Gwynne DT, Anderson GL (2005) Radiotelemetry reveals differences in individual movement patterns between outbreak and non-outbreak Mormon cricket populations. Ecol Entomol 30:548–555
Lüdecke D, Ben-Shachar M, Patil I, et al (2021) performance: An R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139. https://doi.org/10.21105/joss.03139
Lüdecke D (2022) _sjstats: Statistical Functions for Regression Models (Version 0.18.2)_. https://cran.r-project.org/package=sjstats
Mařan J (1958) Eine neue Art der Gattung Isophya Br. W. aus der Tschechoslowakei, Orthoptera - Tettigoniidae. Acta Entomol Musei Natl Pragae XXXII:5–24
Matenaar D, Bröder L, Bazelet CS, Hochkirch A (2014) Persisting in a windy habitat: population ecology and behavioral adaptations of two endemic grasshopper species in the Cape region (South Africa). J Insect Conserv 18:447–456. https://doi.org/10.1007/s10841-014-9654-4
McCartney J, Armstrong DP, Gwynne DT et al (2006) Estimating abundance, age structure and sex ratio of a recently discovered New Zealand tusked weta Motuweta riparia (Orthoptera, Anostostomatidae), using mark-recapture analysis. N Z J Ecol 30:229–235. https://doi.org/10.5422/fordham/9780823244607.003.0004
Morton AC (1982) The effects of marking and capture on recapture frequencies of butterflies. Oecologia 53:105–110
Narisu J, Lockwood A, Schell SP (1999) A novel mark-recapture technique and its application to monitoring the direction and distance of local movements of rangeland grasshoppers (Orthoptera: Acrididae) in the context of pest management. J Appl Ecol 36:604–617. https://doi.org/10.1046/j.1365-2664.1999.00421.x
Nuhlíčková S, Svetlík J, Krištín A (2017) First record of keeled plump bush-cricket (Isophya costata Brunner von Wattenwyl, 1878) (orthoptera, tettigoniidae) in Slovakia. Trav Du Museum Natl D’histoire Nat Grigore Antipa 60:435–440. https://doi.org/10.1515/travmu-2017-0009
Nuhlíčková S, Svetlík J, Šibíková M et al (2021b) Current distribution, microhabitat requirements and vulnerability of the Keeled Plump Bush-cricket (Isophya costata) at the north-western periphery of its range. J Insect Conserv 25:65–76. https://doi.org/10.1007/s10841-020-00280-w
Nuhlíčková S, Svetlík J, Krištín A, et al (2021a) Rozšírenie a ekológia endemickej kobylky Isophya beybienkoi: prvé poznatky pre zabezpečenie ochrany kriticky ohrozeného druhu. In: Ochrana prírody - Rozšírené abstrakty príspevkov z konferencie. Štátna ochrana prírody SR, Banská Bystrica, pp 22–23
Orci KM, Szövényi G, Nagy B (2001) Description of the song of Isophya beybienkoi (Orthoptera, Tettigonioidea). Biol 56:489–495
Poniatowski D, Fartmann T (2011a) Does wing dimorphism affect mobility in Metrioptera roeselii (Orthoptera: Tettigoniidae)? Eur J Entomol 108:409–415. https://doi.org/10.14411/eje.2011.052
Poniatowski D, Fartmann T (2011b) Dispersal capability in a habitat specialist bush cricket: The role of population density and habitat moisture. Ecol Entomol 36:717–723. https://doi.org/10.1111/j.1365-2311.2011.01320.x
R Core Team (2023) _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria
Rada S, Štepánová L, Losík J, et al (2015) How does Oedipoda germanica (Orthoptera: Acrididae) cope on the northern edge of its distribution? A demographical study of a completely isolated population. Eur J Entomol 112:486–492. https://doi.org/10.14411/eje.2015.062
Růžičková J, Elek Z (2021) Recording fine-scale movement of ground beetles by two methods: Potentials and methodological pitfalls. Ecol Evol 11:8562–8572. https://doi.org/10.1002/ece3.7670
Sielezniew M, Kostro-Ambroziak A, Kőrösi Á (2020) Sexual differences in age-dependent survival and life span of adults in a natural butterfly population. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-66922-w
Tang Y, Horikoshi M, Li W (2016) ggfortify: Unified Interface to Visualize Statistical Result of Popular R Packages. R J 8:474–485
Therneau TM, Grambsch PM (2000) Modeling Survival Data: Extending the Cox Model. Springer, New York
Therneau T (2022) A Package for Survival Analysis in R. R Packag. version 3.4–0. https//cran.r-project.org/package=survival
Vadkerti E, Szövényi G (2005) Habitat preference of four protected bush-cricket species (Orthoptera, Phaneropteridae, Isophya) in South Hungary. Biol 60:545–549
Van Dyck H, Baguette M (2005) Dispersal behaviour in fragmented landscapes: Routine or special movements? Basic Appl Ecol 6:535–545. https://doi.org/10.1016/j.baae.2005.03.005
Voigt CC, Michener R, Kunz TH (2005) The energetics of trading nuptial gifts for copulations in katydids. Physiol Biochem Zool 78:417–423. https://doi.org/10.1086/430224
Wagner A (2015) Handlungsbedarf Isophya costata, Breitstirnige Plumpschrecke. Knollconsult Umweltplanung ZT GmbH, Wienna
Watts C, Empson R, Thornburrow D, Rohan M (2012) Movements, behaviour and survival of adult Cook Strait giant weta (Deinacrida rugosa; Anostostomatidae: Orthoptera) immediately after translocation as revealed by radiotracking. J Insect Conserv 16:763–776. https://doi.org/10.1007/s10841-012-9461-8
Wedell N, Ritchie MG (2004) Male age, mating status and nuptial gift quality in a bushcricket. Anim Behav 67:1059–1065. https://doi.org/10.1016/j.anbehav.2003.10.007
Weyer J, Weinberger J, Hochkirch A (2012) Mobility and microhabitat utilization in a flightless wetland grasshopper, Chorthippus montanus (Charpentier, 1825). J Insect Conserv 16:379–390. https://doi.org/10.1007/s10841-011-9423-6
Whitman DW (2008) The significance of body size in the Orthoptera: a review. J Orthoptera Res 17:117–134. https://doi.org/10.1665/1082-6467-17.2.117
Wickham H (2016) Elegant Graphics for Data Analysis. Springer-Verlag, New York
Acknowledgements
Our special thanks go to the Mohammed bin Zayed Species Conservation Fund for supporting this study. Our great thanks also go to the SmartWater® Foundation, which kindly provided the fluorescent dyes used for this research. We thank the Slovak Karst National Park Administration for logistical support and permits. Peter Tuček, Stanislava Pekarová and Michaela Erdélska helped us in the field work. This study was also partially supported by the Slovak Scientific Grant Agency VEGA (2/0097/23 and 2/0097/22).
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This study was funded by the Mohammed bin Zayed Species Conservation Fund (project no. 202524398) and was partially supported by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences (VEGA) (project no. 2/0097/23 (AK, BJ, PK) and no. 2/0097/22 (SN, JS).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by [Soňa Nuhlíčková], [Ján Svetlík], [Anton Krištín] and [Benjamín Jarčuška]. Data analysis was performed by [Benjamín Jarčuška] and [Peter Kaňuch]. The first draft of the manuscript was written by [Soňa Nuhlíčková] and [Anton Krištín]. All authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript. Conceptualisation: [Soňa Nuhlíčková]; Methodology: [Soňa Nuhlíčková], [Ján Svetlík], [Anton Krištín], [Peter Kaňuch] and [Benjamín Jarčuška]. Formal analysis and data investigation [Benjamín Jarčuška] and [Peter Kaňuch]; Writing – original draft preparation: [Soňa Nuhlíčková] and [Anton Krištín]; Writing – review and editing: [Soňa Nuhlíčková], [Ján Svetlík], [Anton Krištín], [Peter Kaňuch] and [Benjamín Jarčuška]; Funding acquisition: [Soňa Nuhlíčková].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval (include appropriate approvals or waivers)
All authors declare that they did not collect threatened arthropods from the study areas. All marked animals were carefully returned to the place of their collection.
Consent to participate (include appropriate statements)
Not applicable.
Consent for publication (include appropriate statements)
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the unstructured Abstract, the heading 'Introduction' was inadvertently added in the beginning of the Abstract. The Abstract has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
10841_2023_529_MOESM1_ESM.xlsx
Supplementary file1 (XLSX 10 KB) Online Resource 1. The number of controls, date, captured individuals and recaptured events at the two experimental sites. The first capture session is indicated with *. Explanations: ES1 – experimental site 1; ES2 – experimental site 2; Start – time of the first captured individual; End – time of the last captured individual; Cap M – total number of males captured; Cap F – total number of females captured; Recap M – total number of males recaptured; Recap F – total number of females recaptured.
10841_2023_529_MOESM2_ESM.xlsx
Supplementary file2 (XLSX 22 KB) Online Resource 2. List of all captured, marked and recaptured individuals (n = 192) found in this study. Explanations: ID – identification code; M – male; F – female; ES1 – experimental site 1; ES2 – experimental site. Due to the protection of critically endangered species, we do not provide exact coordinates in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nuhlíčková, S., Svetlík, J., Kaňuch, P. et al. Movement patterns of the endemic flightless bush-cricket, Isophya beybienkoi. J Insect Conserv 28, 141–150 (2024). https://doi.org/10.1007/s10841-023-00529-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00529-0