Abstract
Photinus signaticollis Blanchard, 1846 (Coleoptera: Lampyridae) is a firefly native to South America and recently established in Europe. Since 2016, this firefly has colonized the northeastern part of the Iberian Peninsula and crossed the Pyrenees to reach southern France in 2019. The larvae of this firefly feed on earthworms, so a high density of this species could negatively impact this key group in soil processes and agriculture. The precise extent of colonization, the environmental niche and the potential range expansion in non-native areas are currently unknown. Using species distribution models, we have found the high suitability areas across Europe where P. signaticollis may become established if introduced. Interestingly, using only South American records and associated conditions modelling it can be strongly predicted where the species is currently found in Europe. Despite a few propagules of P. signaticollis detected in very unsuitable areas of Spain were no longer detected after their initial observation, the climatic niche overlap between South America and Europe populations appeared to be very low. In our case, this pattern is more likely to reflect a high unfilled niche rather than a niche expansion or niche shift, because many occupied areas in South America possess a climate not occurring in Europe. Among the considered variables, we furthermore found that only the temperature seasonality appeared to be the same in both native and European areas and relevant in the models, suggesting that it may represent the main limiting factor for establishing this firefly.
Implications for insect conservation
The introduction and population expansion of a non-native firefly is a novel event in European communities and the effects are unpredictable: through their interspecific competition with native fireflies and possible interactions with other insects, they could disrupt fragile surface balances, and their feeding on earthworms could potentially affect soil communities and processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are among the most important drivers of biodiversity loss (IPBES 2019). In addition to the natural movement of fauna and flora (Masters and Norgrove 2010), globalization and increased movements of goods lead to a rapid increase in biological colonization by foreign species and potential invasions (Mormul et al. 2022). Invasion events by exotic species can cause direct or indirect damage to native fauna and flora or even human activities and health by producing pests or diseases (Mooney & Hobbs 2000; Crowl et al. 2008; Pyšek and Richardson 2010). Often, the impact of exotic species is long-term, produced by not-monitored species or caused by organisms of low importance from the point of view of human activity and thus not considered invasive sensu stricto. For these reasons, detecting and investigating all types of exotic species is of utmost importance to implement curative measures as soon as possible or to exercise extreme caution and prevention in areas not yet colonized (Kim et al. 2006; Hulme 2009).
An important part of studying exotic and invasive species is investigating their ecology in their area of origin and comparing it with that shown in the occupied non-native areas. Hence, extrapolating the ecological requirements of the species, such as the climatic niche, to colonized or potentially colonized areas will subsequently allow to predict the potential risk areas and undertake curative actions early (Thuiller et al. 2005; Jiménez-Valverde et al. 2011). However, the reality is that for many insect species there is a serious lack of ecological information in invaded or alien areas as well as in native areas. Therefore, studies investigating this issue help both to generate new knowledge and to make possible management decisions if the alien species behaves as an invader or pest.
Here, we focus on an exotic South American firefly species in Europe, Photinus signaticollis Blanchard, 1846 (Coleoptera: Lampyridae) (Figure S1) that thus far is colonizing some territories of the Iberian Peninsula (northeast and some inland areas) and southeastern France (Oriental Pyrenees), on both sides of the Pyrenees (Koken et al. 2022). This species was originally identified and described from Catalonia in 2018 as Photinus immigrans Zaragoza-Caballero and Viñolas (2018), and was presumed to be native to Central or North America. However, the species was eventually synonymized with the species P. signaticollis from Argentina and Uruguay by comparing the genitalia of South American and European specimens (Koken et al. 2022).
At least in Europe, P. signaticollis has a dispersal distance of 6 ± 3.5 km/year, prefers pasture areas to other types of lands, and shows two peaks of adult presence, a smaller one at the end of May and a larger one at early August (in South America, at the end of February and November), apparently avoiding periods with higher temperatures (in Europe, July and early August) (Koken et al. 2022).
In addition, preliminary data from captive breeding of the species indicate that females laid eggs on the soil, more specifically on decaying leaves, litter and grass stems or inside thick well-developed and moist root systems of pastures where the larvae seem to thrive (De Cock unpublished; Koken et al. 2022). In captivity, the larvae always hide from light and reside in layers with very humid conditions (De Cock, unpublished data). Interestingly, the larvae of one brood, grown under the same conditions (room temperature 18–26 °C, same access to food), can develop fast, i.e., in eight months, or take longer and become adults after more than one year. Metamorphosis to adults in Europe happens in Spring (April-May) and late Summer (August-September). This strategy, with a rather long adult life span (3–4 weeks), allows offspring of one brood to “colonize” over warm seasons (Spring – early Autumn) but also over more years (De Cock, unpublished data).
The larva of P. signaticollis is a predator of earthworms and seems not to be trophically associated with slugs and snails, thus probably not competing at the food resource level with most European native glow-worms and fireflies (Koken et al. 2022), except of the Photinini genera Phosphaenus and Phosphaenopterus that also predate on earthworms (De Cock 2000; Cock 2009; Nunes et al. 2021). However, the introduction of this new taxon into the European biological communities may have consequences for the native oligochaete fauna, especially if the exotic firefly occurs in high densities, negatively impacting this key invertebrate group known to be very important in soil processes and agriculture (Edwards 2004; Bertrand et al. 2015).
Here, (1) we describe and compare the climatic niche of P. signaticollis in both its native and non-native areas to evaluate the degree of niche overlapping and shift, and (2) we attempt to characterize the potential distribution of P. signaticollis using species distribution models (SDMs) (both in native and colonized areas), and in particular we identified the most suitable areas for colonization of this exotic firefly in Europe based on climatic variables. Through this methodology, we outlined the potentially most risky areas due to the establishment of this species.
Materials and methods
Occurrence points
To collect all the available geo-referenced data of P. signaticollis from South America (the native range), the Global Biodiversity Information Facility (GBIF) (http://www.GBIF.org) was consulted. The available data for Europe (the colonized range) were extracted from the citizen science platforms Biodiversidad Virtual (www.biodiversidadvirtual.org) and iNaturalist (www.inaturalist.org) (data incorporated in GBIF). In this region (especially in Spain and France), there are citizen science projects working to collect these records: “Grup Cucadellum”, “Observatoire des vers Luisants et des lucioles” and “Gusanos de Luz”. Both datasets were compiled in Koken et al. (2022) (Table S1, Figure S2). All occurrence records used in our analyses have been corroborated or identified by the authors of this article by photographic identification. To avoid spatial bias and contagion in highly sampled areas, we sifted all the data from both areas into a 1 × 1 km grid, thus obtaining the centroids of the grids with presence points for the species distribution models. New occurrence points of this firefly in South America (Table S2) obtained during the preparation of this manuscript have been used to perform an external evaluation analysis of the final model.
Data variables
The 19 bioclimatic variables of the current climate of WorldClim database version 2.1 (http://www.worldclim.org) were extracted with 30 s cell sizes (i.e., grids of 1 × 1 km) and fitted into two spatial frameworks: the Neotropic (i.e.,: South America, the native range) and the Western Palearctic (i.e., Europe, the colonized range).
The bioclimatic variables present data from 1970 to 2000 and are widely used to predict the potential distribution of species by reflecting temperature and precipitation patterns and their variation since these are overall ecologically meaningful for species (Araújo and Guisan 2006; Peterson et al. 2011). The work scheme of Polidori et al. (2021)d mez et al. (2022) was applied, considering that the climatic variables were strongly correlated. A hierarchical cluster analysis was performed, resulting in a dendrogram showing the similarity in the two studied areas (native and the colonized) among all the studied variables (Dormann et al. 2013). We used Ward-clustering based on the correlation matrix, one of the most used methods to obtain distances among the variables (Harrell 2015). The chosen distance threshold to form the clusters was set at 0.3, i.e., less than 70% correlation (following Polidori et al. 2021). To select the variables included in the same cluster, we followed the next statements: (1) the one that more optimally discriminates between habitable and non-habitable areas in a previous simple environmental coverage model (see below), or (2) the one that makes the most biological sense a priori for this species of firefly, (3) the most derived variable (Figure S3).
The final set of selected variables included: temperature seasonality (bio4), annual temperature range (bio7), mean temperature of wettest quarter (bio8), mean temperature of warmest quarter (bio10), annual precipitation (bio12), and precipitation of coldest quarter (bio19) (Figure S3).
Species distribution modeling
We estimated the climate-based potential distribution of P. signaticollis through two approaches: (1) a simple environmental coverage model and (2) ensemble modeling.
To determine the similar areas amongst all present and to be able to project the climatic conditions of the native area over the colonized zones (and vice versa), a simple environmental coverage model was performed. This model shows by means of an index (simple environmental coverage model index, SECMi) ranging from 0 to 19 (for the 19 variables used in WorldClim), the habitability of a territory (i.e., the degree of resemblance to the area conformed by the presences). The SECMi is calculated by the sum of each environmental variable transformed into binary (0–1), where 1 represents areas with values within the range of extremes for that variable describing the species’ presence, and 0 represents areas with values higher or lower than the range of extremes for that variable. The highest value (i.e., SECMi = 19) indicates the most habitable area, and the lower values (SECMi < 19) are considered non-habitable areas (Gil-Tapetado et al. 2022).
To investigate the spatial ecology of P. signaticollis we used six different algorithms through the biomod2 library (Thuiller et al. 2019): Generalized Linear Model (GLM), Generalized Additive Model (GAM), Artificial Neural Network (ANN), Classification Tree Analysis (CTA), Random Forest (RF) and Maximum Entropy (MaxEnt). The final ensemble model is based on the average of 72 individual models (i.e., 12 iterations × 6 algorithms) and was used to predict the potential distribution of the studied firefly.
The background and pseudoabsence construction was based on the simple environmental coverage model. Habitable areas (SECMi = 19) were used to establish the background points, whereas areas that did not fulfil at least one of these variables (SECMi < 19) were used to establish the pseudoabsence points (Gil-Tapetado et al. 2018; Polidori et al. 2021).
Presence, pseudoabsence and background data were split in 75/25% to generate an external “Area Under the receiver operating characteristic Curve” (AUC) evaluation for the final models, independently of the internal AUC evaluations of each model generated by biomod2. A total of 60 individual models were tested with their individual AUC evaluation, choosing only the models with AUC > 0.7 (i.e., good to excellent performance of the model following the scale of Thuiller (2003). All the models were validated and fulfilled this condition and were used to calculate the final ensemble average model.
Finally, the ensemble models were evaluated through the external AUC test with 25% of the data. Also, the occurrences collected during the preparation of this manuscript (see Occurrence points section) were used to perform another external evaluation through an AUC test with this external dataset and an iteration of the same number of randomly generated pseudoabsences with 100 replicates, obtaining an average AUC value. The Cut-off value of the final ensemble model was calculated with the total sum of squares (TSS) of the ensemble model (Europe = 0.713; South America = 0.581) to establish the areas of the presence of P. signaticollis.
Variable selection and species distribution models were carried out using the R v 3.5.0 program through RStudio Software v 1.1.453 (Rstudio Team 2015). Background and pseudoabsences point generation and model maps were performed in ArcGIS for Desktop v 10.3 (ESRI 2014).
Distribution and ecological analyses
We analyzed the potential distributions of P. signaticollis in both territories of interest using an analysis of variance (ANOVA), comparing the areas of presence and absence in the native and colonized areas and the areas of presence with each other. In this way, we were able to evaluate whether the presence of this firefly in Europe varies ecologically with respect to its native area.
Finally, we compared the two areas through a principal component analysis (PCA) of the occurrences in South America and Europe, another PCA of 10,000 random points in the high suitability areas of each ensemble model in the two areas and a radar chart with the mean value of the previous points by each variable used in the models. Likewise, the niche overlap, equivalency and similarity of P. signaticollis in its native and colonized areas were calculated (Wiens and Graham 2005; Broennimann et al. 2012; Guisan et al. 2014), obtaining the expansion, stability and unfilling of the species niche. Niche overlap between native and colonized range was calculated using Schoener’s D index (Schoener 1970), which varies from zero (no overlap between niches) to one (total overlap). We then followed Broennimann et al. (2012) to niche equivalency (i.e., whether niche overlap is constant when randomly reallocating the occurrences of both entities among the native and colonized areas) and similarity (i.e., whether the overlap between observed niches in native and colonized areas is different from the overlap between the observed niche in one area and randomly selected niches from the other area). The null hypothesis of niche equivalency cannot be rejected if the observed value of D falls within the density of 95% of 1000 simulated values while the species occupies environments in both of its ranges that are more similar to each other than expected if the observed overlap is greater than 95% of 1000 simulated values (Broennimann et al. 2012). We follow Polidori and Sánchez-Fernández (2020) to perform these analyses using the ecospat package (Di Cola et al. 2017) in R-Studio.
Results
Simple environmental coverage models (Fig. 1) show that P. signaticollis has the highest SECMi values (habitable areas) in the Pampean region of South America and around the Mediterranean and Atlantic coasts (ignoring those of the Cantabrian Sea) in Europe. These maps show that the habitable areas in South America, its native zone, are found in a larger territory (Fig. 1B) than in the colonized zone (Fig. 1A). This indicates that the conditions of the colonized areas are more restricted than the conditions of their native area (Table 1). Considering the total conditions of the species (with all the occurrences in both areas), it is observed that in South America, there are few changes in the most habitable areas, while in Europe, there are potentially more habitable territories (Fig. 1C).
The evaluation of the ensemble model for the AUC test with 25% of the data indicated an optimal performance of the model for South America (1.00) and Europe (1.00) (Figure S4). The AUC test with the South American ensemble model with an external dataset also shows an excellent model performance (0.996). The ensemble model indicates that the highly suitable areas for this firefly in South America are located in the temperate climate zones with warmer summers of La Pampa, around the mouth of the Río de la Plata, and the El Chaco areas (Fig. 2A). On the other hand, in Europe, the high suitability areas (i.e., risk areas where it is more likely that P. signaticollis can settle if it arrives) are located on the Northeastern Mediterranean coasts of the Iberian Peninsula (in Catalonia and the north of the Valencian Community) and scattered areas of inland France, Northern France and Belgium (West Flanders), Italy (between Livorno and Piombino) and England, all zones where this species has not yet been reported (Fig. 2B). Inland areas of the Iberian Peninsula (La Rioja and Extremadura) show low suitability for this species.
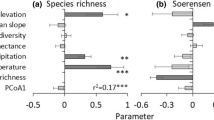
Differences among variables that consider the high- and low-suitability areas of the native and colonized area (Fig. 3) indicated that, in South America, the highly suitable areas of P. signaticollis are characterized by having greater temperature seasonality (bio4) and annual temperature range (bio7), while the rest of the variables (mean temperature of wettest quarter (bio8), mean temperature of warmest quarter (bio10), annual precipitation (bio12), and precipitation of coldest quarter (bio19)) have lower average values (Fig. 3A). On the other hand, in Europe (Fig. 3B), high suitability areas are characterized by lower temperature seasonality and annual temperature range and high annual precipitation and precipitation of coldest quarter than in South America. The most important variables in South America are temperature seasonality and annual temperature range, while in Europe, it is also temperature seasonality, together with the precipitation of coldest quarter (Table 2).
A comparison of the highly suitable areas of South America and Europe (Fig. 4) indicates significant differences between the used variables except for temperature seasonality. In the cases of annual temperature range, mean temperature of wettest quarter, mean temperature of warmest quarter and annual precipitation, there are higher mean values in South America than in Europe. In contrast, the precipitation of coldest quarter is higher in Europe than in South America. PCAs of empirical occurrences (Fig. 5A) and high suitability areas (Fig. 5B) from South America and Europe to observe the degree of niche overlapping indicate a very low niche overlap between fireflies in native and colonized areas. This low niche overlap is also demonstrated by analyses of niche overlap parameters (Table 3), indicating that niche expansion and unfilling were higher than stability in both empirical occurrences and high suitability areas. In addition, no significant results were found for both niche equivalency and niche similarity tests with the occurrence data but with the ensemble models data, indicating a greater niche similarity in these last analyses. The comparison between the values of the variables used in the P. signaticollis models (Fig. 5C) shows that the values of the presence variables of this firefly in Europe are lower than those in South America, except in the case of the precipitation of coldest quarter.
Simple environmental coverage model of P. signaticollis in South America (left) and Europe (right) considering the range of maximal and minimal values of the occurrences in (A) Europe, (B) South America, (C) Both areas. Values of the map are related to the simple environmental coverage model index (SECMi) from 19 (all environmental conditions match with the occurrences) to 0 (no conditions match with the occurrences)
A. Ensemble model of Photinus signaticollis in South America (native range) B. Ensemble model of this species in Europe and Northern Africa. Values represent the suitability of each ensemble model. Cut-off values of the ensemble models (brown bar) for South America (0.581) and Europe (0.713) are indicative areas which are also suitable and/or potentially colonizable by P. signaticollis. In the top right corner, a photograph of a male individual of P. signaticollis in Maureillas-Las-Illas, France, on 13 Jun. 2022. Author of the photograph: Marcel Koken
A. Graph of principal component analysis of the occurrences of P. signaticollis in South America and Europe considering the variables used in the ensemble model. B. Graph of principal component analysis of random points in the high suitability areas of the ensemble models of P. signaticollis in South America and Europe considering the variables used in the ensemble model. C. Radar chart and comparison of the mean values of presence points by each variable used in the ensemble model of Photinus signaticollis in South America and Europe (temperature seasonality and annual precipitation are divided by 100 and precipitation of the coldest quarter by 10 to improve the display of the graph)
Discussion
Photinus signaticollis in its native South America is mainly distributed in the temperate zones of Argentina and Uruguay, which have – at least in some areas – very similar climatic characteristics to those found on the western Mediterranean coast. This climatic similarity seems to have allowed this firefly to colonize the western Mediterranean, as the species is pre-adapted to the same environmental conditions. According to our results, seasonal temperature seems to be the same between native and invaded areas. The climatic suitability of foreign areas (Jeschke and Strayer 2008) and an apparent lack of native predators/parasitoids (Colautti et al. 2004) are likely responsible for the detection of large numbers of individuals of P. signaticollis in Europe in the last years (2016–2023) since the first European records (Koken et al. 2022).
More in detail, both native and colonized areas are considered climatically temperate (Beck et al. 2018), with South American zones being considered as Humid Subtropical (Cfa), and European zones as typical Mediterranean (Csa) (according to the Köppen–Geiger climate classification). Although there may be differences in the types of climates, the area where P. signaticollis has currently been detected in the Iberian Peninsula (specifically in Girona) is one of the areas with the highest rainfall on the western Mediterranean shores (Zittis et al. 2021). The climatic similarity between native and foreign areas is one of the keys for species to colonize the latter (Liu et al. 2020) since, especially for ectotherms, they are adapted to certain temperature and humidity conditions that are part of their ecological niche (Venette et al. 2010; Willmer and Stone 1997). The climatic niches for P. signaticollis in native and colonized areas differ significantly for all climatic variables except for temperature seasonality, showing either a niche expansion of the species or a niche unfilling with South American conditions that are present in Europe. Also, our models indicate that the species has high suitability values in some areas of Europe outside the ranges of South American variables, i.e., areas with lower annual temperature range or lower and higher mean temperature of wettest quarter. The species could have a niche expansion in Europe in these areas, but it would be unlikely and critical that it could make a niche shift in less than ten years since its introduction, although similar cases in other species have been reported (Hill et al. 2017; Polidori and Sánchez-Fernández 2020). On the other hand, the difference between the niches of the native and colonized zones may be due to an unfilling niche, i.e., part of the climatic conditions of South America are missing in Europe, so in the colonized continent, this species cannot fill the niche it has in the native continent. All the results indicate that this species may extend its distribution across the European continent, colonizing new areas. The areas of P. signaticollis in Europe are less humid and colder than those of South America but not for the variable temperature seasonality, i.e., temperature stable areas. In addition, for the models of the two areas, this variable is the most important in almost all model algorithms, denoting the relevance of temperature stability, which the species seems to require (see Table 2). These results suggest the hypothesis that although there are differences between the species niches in the two areas, these differences are smaller than the real species tolerance ranges (physiological ranges that have not yet been tested), except for temperature seasonality or stability, which appears to be a limiting variable for P. signaticollis, which is in line with the phenological and observations (see Introduction). To explore this question further, when we examine the data separately, we obtain a priori different temperature seasonality results for the native and colonized areas (see Fig. 3). On the one hand, in South America P. signaticollis only occurs in areas with high temperature seasonality (compared to the native range), whereas in Europe it occurs in areas with low temperature seasonality (compared to the colonized range). If we compare the two areas, we see that there are no significant differences between them, so they have similar conditions (even if they appear to be relatively contradictory). With this global view of the niche of P. signaticollis, we obtain that in the native area there is the “lower limit” of temperature seasonality conditions suitable for this firefly, whereas in the colonized area there is the “upper limit”, forming both the “global limits” of the distribution limited by temperature seasonality.The humid subtropical (humid temperate) areas of the Iberian Peninsula close to the European distribution of P. signaticollis (inner Catalonia) are typically very rainy and relatively cold (AEMet 2018), unlike the subtropical Pampean areas. The European climatic conditions indicated by our models are like those in the native area, except for the rainfall in the coldest quarter, which is higher in the colonized area. However, this may not negatively affect the species, as the life cycle of the species shows that the animals are inactive or in a larval stage beneath the ground in winter (Koken et al. 2022), where climatic conditions have less influence than above ground.
As the simple coverage models show (Fig. 1), some areas encompass the same climatic conditions in the two areas of the firefly’s distribution, although the mean values vary. Using the climatic conditions of South America in Europe, the area with the highest SECMi is found in Spain and France where P. signaticollis is already found. This comparison of climatic niches makes it possible to observe some similarities between the native and colonized areas, obtaining the areas where a species can be established since the abiotic conditions of the species’ fundamental niche are similar (Soberón 2010), even if the niches do not overlap. This could also indicate that P. signaticollis is in a climatically restricted area in Europe, although it possesses the adaptations to thrive there. If the biotic conditions are met, i.e., if the necessary resources exist to survive and if there are no antagonistic species with which to compete (fireflies have a drastic decrease in their populations worldwide, including the Western Palearctic (Lewis et al. 2020) or is predated/parasitized (facilitated by the enemy release hypothesis (Colautti et al. 2004), the area would be suitable for this exotic species. Also, note that the coloration pattern of P. signaticollis adults is quite reminiscent of that of male adult Nyctophila reichii Du Val, 1859, an abundant, quite colorful firefly species present throughout Spain and South-Western France (Guzmán and De Cock 2011; Constantin 2014). Knowing that this local species uses defensive chemicals and aposematic displays (Berger et al. 2021), the “newcomer” species may benefit from less predation upon arrival through the mechanisms resembling Batesian or Müllerian mimicry, even if P. signaticollis has not shared a common natural history and joint evolution with the native species.
Our potential distribution models for P. signaticollis show that there are suitable areas not yet colonized by this species in Europe, mainly in the French territories of the Cher, Nièvre and Allier departments and the Paris area, the Italian coast of Tuscany and Sicily, Belgium (i.e., Province of West Flanders), and inland areas of England (see Fig. 2B). These areas should be particularly monitored to control the possible appearance of this exotic firefly due to their high suitability in abiotic factors and, thus, high probability of establishment.
Our models show low suitability for P. signaticollis establishment in the Spanish inland areas in contrast to the coastal zones of Girona. Here suitability is high, and an already well-established population of this firefly is present. In this region, this firefly appears near cornfields with a special irrigation technique for water overflowing in winter. This may also have an extra beneficial influence on the settling of P. signaticollis in this area. Coincidentally, in coming years, this agronomical technique will be abandoned, which could negatively affect the population of Girona and harm its expansion throughout Europe (Ramón Guzmán personal communication). However, a similar practice will continue to exist in the “Pyrénées Orientales”, where old small canals disperse the waters of a small river over a wide range, and fields are regularly flooded during the hot season (Marcel Koken unpublished). Photinus signaticollis is present in a daily water-sprayed garden in Extremadura, the watering possibly explaining the long-term maintenance of this small population in this less suitable area, and it was observed once in La Rioja, in inland Spain (Koken et al. 2022). In Extremadura, the species does not seem to disperse, and in La Rioja, it has thus far not been detected again, suggesting that it has not propagated or established in these areas, in contrast to what happened in the Girona-France population. This may indicate that colonization did not occur because of the insect’s inability to establish a lasting population due to unfavorable climatic conditions, and these records were only of propagules that failed to prosper. Although we do not know the propagule pressures in these areas that have a direct impact on the establishment (Lockwood et al. 2004), the climatic conditions shown in our model are clearly non favorable, and this fact will still be an important factor making it difficult or impossible for this exotic firefly to develop in these areas.
Photinus signaticollis is yet an additional case of an introduction event of an exotic species to Europe from the Pampean region, such as the Argentine ant (Linepithema humile (Mayr, 1868) Hymenoptera: Formicidae) (Human and Gordon 1996; Roura-Pascual et al. 2004) or the palm tree borer (Paysandisia archon (Burmeister, 1880) Lepidoptera: Castniidae) (Muñoz-Adalia and Colinas 2020). These species have all been introduced throughout Europe and other parts of the world due to quarantine problems during the transport of plant materials, such as pots, potting soil, or palm trees themselves, in the case of P. archon. This would be in line with the hypothesis raised by Koken et al. (2022) on the introduction mode of P. signaticollis into the Palearctic by indirect and passive transport of larvae/pupae during the importation of plants with soil from the firefly’s native area.
We carried out this ecological and biogeographical study, which follows previous work by Koken et al. (2020) and suggest that the next logical step will consist in monitoring and studying the impact of this exotic species on native species. This is the only way to quantify the damage and impact that P. signaticollis may have on the native fauna to develop control programs. In addition, the taxonomy and ecology of fireflies are not fully resolved, and the introduction of P. signaticollis into non-native areas may affect the biodiversity of Lampyridae in unforeseen ways. This exotic species should be considered a neglected invasive species and future studies will have to establish whether it can present a risk to native species. However, we are already well aware of its high dispersal potential and its possibly negative impacts on earthworm communities.
References
AEMet (Agencia Española de Meteorología) (2018) Climate maps of Spain (1981–2010) and reference potential evapotranspiration (1996–2016). Available at: https://www.aemet.es/documentos/es/conocermas/recursos_en_linea/publicaciones_y_estudios/publicaciones/MapasclimaticosdeEspana19812010/MapasclimaticosdeEspana19812010.pdf
Araújo MB, Guisan A (2006) Five (or so) challenges for species distribution modelling. J Biogeogr 33:1677–1688. https://doi.org/10.1111/j.1365-2699.2006.01584.x
Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF (2018) Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci data 5(1):1–12
Berger A, Petschenka G, Degenkolb T, Geisthardt M, Vilcinskas A (2021) Insect collections as an untapped source of Bioactive Compounds—Fireflies (Coleoptera: Lampyridae) and cardiotonic steroids as a Proof of Concept. Insects 12(8):689. https://doi.org/10.3390/insects12080689
Bertrand M, Barot S, Blouin M, Whalen J, de Oliveira T, Roger-Estrade J (2015) Earthworm services for cropping systems. A review. Agron Sustain Dev 35:553–567
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG et al (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecol Biogeogr 21(4):481–497
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7(8):721–733
Constantin R (2014) Contribution à l’étude des Lampyridae de France, actualisation de leur distribution et observations en France de Lampyris iberica Geisthardt, Figueira, Day & De Cock, 2008 (Coleoptera, Elateroidea). Le Coléoptériste 17(1):34–44
Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE (2008) The spread of invasive species and infectious disease as drivers of ecosystem change. Front Ecol Environ 6(5):238–246
De Cock R (2000) Rare, or simply overlooked? Practical notes for survey and monitoring of the small glow-worm Phosphaenus hemipterus (Coleoptera: Lampyridae). Belg J Zoo 130:93–101
De Cock R (2009) Biology and behaviour of european lampyrids. Bioluminescence in Focus – A collection of Illuminating Essays (Ed.: Victor Benno Meyer-Rochow). Research signpost, Kerala, India, pp 161–200
Di Cola V, Broennimann O, Petitpierre B, Breiner FT, d’Amen M, Randin C et al (2017) ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography 40(6):774–787
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, García JR, Gruber B, Lafourcade B, Leitao PJ et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Edwards CA (2004) The importance of earthworms as key representatives of the soil fauna. Earthworm Ecol 2:3–11
ESRI 2014. ArcGIS Desktop, V. 10.3. Available at: http://desktop.arcgis.com/es/desktop/
Gil-Tapetado D, Gómez JF, Cabrero-Sanudo FJ, Nieves-Aldrey JL (2018) Distribution and dispersal of the invasive asian chestnut gall wasp, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), across the heterogeneous landscape of the Iberian Peninsula. Eur J Entomol 115:575–586
Gil-Tapetado D, Soria CD, Gómez JF, Sesma JM, Cabrero‐Sañudo FJ (2022) Aridity could have driven the local extinction of a common and multivoltine butterfly. Ecol Entomol 48(1):40–54
Gómez SR, Gil-Tapetado D, García‐Gila J, Blasco‐Aróstegui J, Polidori C (2022) The leaf beetle Labidostomis lusitanica (Coleoptera: Chrysomelidae) as an Iberian pistachio pest: projecting risky areas. Pest Manag Sci 78(1):217–229
Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C (2014) Unifying niche shift studies: insights from biological invasions. Trends Ecol Evol 29(5):260–269
Guzmán JR, De Cock R (2011) The biology and distribution of glow-worms (Coleoptera: Lampyridae) in Spain. Lampyrid 1:22–31
Harrell FE Jr (2015) Regression modeling strategies – with applications to Linear Models, logistic regression, and Survival Analysis. – Springer Nature Switzerland 582 pp
Hill MP, Gallardo B, Terblanche JS (2017) A global assessment of climatic niche shifts and human influence in insect invasions. Global Ecol Biogeogr 26(6):679–689
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46(1):10–18
Human KG, Gordon DM (1996) Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecol 105(3):405–412
IPBES (2019) Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy. Platform on Biodiversity and Ecosystem Services. IPBES Secretariat, Bonn, Germany
Jeschke JM, Strayer DL (2008) Usefulness of bioclimatic models for studying climate change and invasive species. Ann NY Acad Sci 1134(1):1–24
Jiménez-Valverde A, Peterson AT, Soberón J, Overton JM, Aragón P, Lobo JM (2011) Use of niche models in invasive species risk assessments. Biol Invasions 13(12):2785–2797
Kim CS, Lubowski RN, Lewandrowski J, Eiswerth ME (2006) Prevention or control: optimal government policies for invasive species management. Agr Resour Ec Rev 35(1):29–40
Koken M, Guzmán-Álvarez JR, Gil-Tapetado D, Romo-Bedate MA, Laurent G, Rubio LE et al (2022) Quick spreading of populations of an exotic firefly throughout Spain and their recent arrival in the French Pyrenees. Insects 13(2):148
Lewis SM, Wong CH, Owens A, Fallon C, Jepsen S, Thancharoen A et al (2020) A global perspective on firefly extinction threats. Bioscience 70(2):157–167
Liu C, Wolter C, Xian W, Jeschke JM (2020) Most invasive species largely conserve their climatic niche. P Natl Acad Sci USA 117(38):23643–23651
Masters G, Norgrove L (2010) Climate change and invasive alien species. UK: CABI Working Paper 1:30
Mooney HA, Hobbs RJ (2000) Invasive species in a changing world. Island Press, Washington DC, p 384
Mormul RP, Vieira DS, Bailly D, Fidanza K, da Silva VFB, da Graça WJ et al (2022) Invasive alien species records are exponentially rising across the Earth. Biol Invasions 24(10):3249–3261. https://doi.org/10.1007/s10530-022-02843-1
Muñoz-Adalia EJ, Colinas C (2020) The invasive moth paysandisia archon in Europe: Biology and control options. J Appl Entomol 144(5):341–350
Nunes V, Figueira G, Lopes LF, Souto P (2021) On the natural history of the Black Winged Firefly, Phosphaenopterus metzneri Schaufuss, 1870 with comparative notes on Phosphaenina (Coleoptera: Lampyridae). Ann Zool 71(3):661–691
Peterson AT, Soberón J, Pearson RG, Anderson RP, Martinez-Meyer E, Nakamura M, Araújo MB (2011) Ecological Niches and Geographic Distributions. Princeton University Press, p 328
Polidori C, Sánchez-Fernández D (2020) Environmental niche and global potential distribution of the giant resin bee Megachile sculpturalis, a rapidly spreading invasive pollinator. Global Ecol Conse 24:e01365
Polidori C, García-Gila J, Blasco-Aróstegui J, Gil-Tapetado D (2021) Urban areas are favouring the spread of an alien mud-dauber wasp into climatically non-optimal latitudes. Acta Oecol 110:103678
Pyšek P, Richardson DM (2010) Invasive species, environmental change and management, and health. Annu Rev Environ Resour 35:25–55
Roura-Pascual N, Suarez AV, Gómez C, Pons P, Touyama Y, Wild AL, Peterson AT (2004) Geographical potential of Argentine ants (Linepithema humile Mayr) in the face of global climate change. P Roy Soc Lond B-Bio 271(1557):2527–2535
RStudio Team, RStudio (2015) R Studio. Integrated Development for R. Inc., Boston, MA. http://www.rstudio.com/
Schoener TW (1970) Nonsynchronous spatial overlap of lizards in patchy habitats. Ecol 51(3):408–418
Soberón JM (2010) Niche and area of distribution modeling: a population ecology perspective. Ecography 33(1):159–167
Thuiller W (2003) BIOMOD–optimizing predictions of species distributions and projecting potential future shifts under global change. Global Change Biol 9(10):1353–1362. https://doi.org/10.1046/j.1365-2486.2003.00666.x
Thuiller W, Richardson DM, Pyšek P, Midgley GF, Hughes GO, Rouget M (2005) Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Global Change biol 11(12):2234–2250
Thuiller W, Georges D, Engler R, Breiner F (2019) Biomod2: ensemble platform for species distribution modeling. R Package Version 3(1):3–7
Venette RC, Kriticos DJ, Magarey RD, Koch FH, Baker RH, Worner SP et al (2010) Pest risk maps for invasive alien species: a roadmap for improvement. Bioscience 60(5):349–362
Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst 36:519–539
Willmer P, Stone G (1997) Temperature and water relations in desert bees. J Therm Biol 22(6):453–465
Zaragoza-Caballero S, Viñolas A (2018) Photinus immigrans sp. nov. (Coleoptera: Lampyridae: Photinini): primer registro del género Photinus en Cataluña, España. Revista Gaditana de Entomología 9(1):273–286
Zittis G, Bruggeman A, Lelieveld J (2021) Revisiting future extreme precipitation trends in the Mediterranean. Weather Clim Extremes 34:100380
Acknowledgements
To Grup Cucadellum-ICHN for its unconditional support, especially Segimon Rovira, Rafael Carbonell and Jordi Clavell for being the driving forces behind the international network in Catalonia, Spain, France, Italy, Belgium and Argentina. Thanks to the observers in the species’ native range; Gusanosdeluz, Grup Cucadellum-ICHN and « l’Observatoire des Vers luisant et des Lucioles » and Lucas Rubio for continually reporting Argentinian Photinus signaticollis observations. Thanks to Silvio Gómes Fritz for his help and support. D.G.-T. was supported by a Margarita Salas University Complutense Madrid contract, financed by the Ministry of Universities with Next Generation funds from the European Union. The title of the manuscript is inspired by the song “Across the Universe” by The Beatles, written by John Lennon and Paul McCartney.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. DG-T was supported by a Margarita Salas UCM contract, financed by the Ministry of Universities with Next Generation funds from the European Union.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
DGT conceived and designed the work. DGT, CP performed the experiments and analyzed the data. DGT, JFG, FJC-S contributed materials/analysis tools. DGT, RDC, MK, FJC-S, CP wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gil-Tapetado, D., Koken, M., De Cock, R. et al. Across the firefly-verse: comparison of niche suitability of an exotic firefly in its native and colonized range. J Insect Conserv 28, 43–56 (2024). https://doi.org/10.1007/s10841-023-00522-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00522-7