Abstract
A diversity of insects can be found at the remains of dead animals (carrion) and they play a vital role in its decomposition and recycling. An emerging global problem with carrion is animal mass mortality events – the sudden, rapid die-off of many animals resulting in a large increase to the localised carrion resource pool. Yet, little is known about how insects respond to sudden and large inputs of carrion. We conducted an experiment in a mountainous alpine region of south-eastern Australia and compared beetle assemblages found at single carcass and mass mortality sites. We also examined the effects of vertebrate exclusion, and decomposition stage on beetles. We found 4,774 beetles representing 146 different species/morphospecies from 17 families. The most abundant species was Saprinus cyaneus cyaneus (Histeridae), and species of Staphylinidae and Silphidae also dominated the fauna, which is typical for necrophilous beetles in Australia. We also found a clear temporal change in beetle assemblages, with abundance and richness peaking during the active decay stage. We found that beetle abundance was greater at single carcasses than mass mortality sites, possibly as an artifact of sampling, and that species richness was similar among these two carcass treatment types. We found no significant effect of vertebrate exclusion on beetles, suggesting that large scavengers in the study system may not influence necrophilous insect communities around carrion.
Implications for insect conservation: Our study highlights the diversity of beetles that can be found at carrion and their similar composition to the fauna found in other areas in the south-east of the Australian continent. Beetles may have reduced abundance at mass mortality sites which could affect their ability to contribute to carrion removal relative to smaller carrion quantities. Further research is required to quantify the role of other insects in carrion removal under a range of natural and mass mortality scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carrion – the remains of dead animals – is a distinct form of necromass in ecosystems and supports an important subsect of biodiversity involved with its breakdown and recycling (Barton et al. 2013a; Benbow et al. 2019; Carter et al. 2007). However, climate and land use changes are altering the inputs of carrion to ecosystems around the world (Barton et al. 2019; Olea et al. 2019). An example of this is the increasing frequency and magnitude of animal mass mortality events (MMEs) occurring around the world as a result of climate change and other anthropogenic factors (Barton et al. 2022; Fey et al. 2015a).
MMEs can lead to substantial inputs of carrion resources in localised areas, with potentially detrimental effects on ecosystems and wildlife through altered nutrient and energy flow or increased disease risk (Barton et al. 2022). Mass mortality events represent significant inputs of carrion resources (Fey et al. 2015a), but it is unclear how biodiversity might respond to MMEs in a range of different ecosystems, or how biodiversity might contribute to the resilience of ecosystems and removal of carrion following disturbance by MMEs (Barton et al. 2022).
The mountainous region of south-east Australia is an ecosystem particularly vulnerable to climate change (Pickering 2007; Worboys et al. 2011), and also contains large numbers of pest animals such as invasive deer, pigs and horses (Cherubin et al. 2019; Driscoll et al. 2019; Mitchell et al. 2015). These two factors increase the likelihood of MMEs through climate-driven mechanisms (e.g., heat stress, wildfire, drought) but high intensity culling practices aimed at reducing pest animal numbers can also effectively simulate an MME.
Our understanding of biodiversity responses to MMEs is limited. Smaller volumes of carrion may be able to be processed by scavengers and rapidly consumed and dispersed. For example, flies have been shown to rapidly accelerate the biomass loss at single carrion sites, with colonisation occurring almost immediately (Lashley et al. 2018). This could occur at MMEs, and flies have been shown to produce ‘rivers’ of larvae at simulated pig mass mortalities in the USA (Lashley et al. 2018). But insect responses in other parts of the world are completely unknown. In some contexts, large MMEs may overwhelm scavenger animals, including insects, resulting in slower decomposition dominated by microbes (Barton et al. 2022). MME decomposition could lead to carcasses persisting in the landscape for longer periods of time, and potentially support larger carrion insect populations, yet this has not been tested (but see Baruzzi et al. 2022; Lashley et al. 2018). Alternatively, insect populations may not be able to respond quickly enough to large carrion quantities but could be sustained over longer time frames as the carrion resources sit in the landscape and decompose more slowly.
To address this knowledge gap, we conducted a study on the responses of beetles to simulated mass mortality events. Our aims were (i) to characterise the beetle assemblages found at carrion in the alpine and sub-alpine region of Kosciuszko National Park, and (ii) to determine if there are any differences in beetle assemblages between single animal carcasses and sites simulating an animal mass mortality. We also (iii) assessed how beetles responded to the absence of vertebrate scavengers, on the basis that vertebrates speed up the decomposition of carrion (Devault et al. 2003). This would mean that, in the absence of vertebrates, there could be a greater amount of carrion biomass left for insect populations to be sustained for longer. To the best of our knowledge, there have been no previous studies of necrophilous beetle assemblages in the Australian alpine and sub-alpine region. This region supports a wide variety of faunal communities with a high degree of endemism (Endo et al. 2015; Happold 2011). The arthropod fauna in this region is also highly diverse, likely including well over 1,000 species across 175 families (Green 2002). Beetles associated with carrion in south-eastern Australia include species in the families Staphylinidae, Histeridae, Scarabaeidae, Silphidae, Trogidae, and Dermestidae, as well other generalist groups such as Carabidae and Leiodidae (Barton and Evans 2017). We predicted that carrion beetle assemblages in the study area would be similarly dominated by species from these families. Because simulated MMEs represent a larger resource, we also predicted they will support higher abundances of beetles, but not necessarily high species richness, than single carcasses. We also predicted that there would be greater abundance of beetles at vertebrate exclusion sites as the absence of vertebrate scavengers would mean that carcasses are not being consumed or decomposed as quickly, resulting in more opportunity for beetles to locate and colonise the carcass. We discuss our findings with consideration of biodiversity conservation and management challenges in the study area.
Materials and methods
Study area
We conducted our study near Kosciuszko National Park, south-eastern Australia between December 2020 and February 2021. Vegetation across our study area ranged from montane forest 1000- 1400 m (moist – wet forests) and tableland forest 400-1000 m (dry – moist forests) (Doherty et al. 2015). Climate in our study area is characterised by a montane climate, an absent dry season with mild summers and snowfall in winter at high elevation (Zhu et al. 2020). During the study period, the mean daily maximum reached 25℃ with a mean daily minimum of 13℃ (Bureau of Meteorology 2021). Our study sites were located in broadly similar vegetation types and elevation that contained large open clearings in which different treatments were placed.
Study design
Our study design used two locations (Chimney Ridge and Waste Point) separated by approximately 21 km, and each location hosting 12 plots. The 12 plots together made a 3 × 2 crossed design of three carcass treatments (no carcass, single carcass, mass mortality carcasses) a vertebrate exclusion treatment (exclusion, no exclusion), with two replicates of each combination of treatments. Each plot was sampled three times over four weeks (weeks 1, 2, and 4), thus giving a full design comprising 2 locations x 12 plots x 3 sample times (n = 72).
Carcass treatment details. We established three carcass treatments at each of the two locations: (i) a control treatment with no carcass, (ii) a single carcass treatment of a single fallow deer (Dama dama), and (iii) a mass mortality treatment with approximately 10 large vertebrate carcasses of a mix of fallow deer and eastern grey kangaroo (Macropus giganteus). Each mass mortality plot was 8 m x 8 m (Fig. 1) and each single carcass site was 1.8 m x 1.2 m. Deer and kangaroo carcasses were obtained from pre-approved culls of pest animals conducted by either local landowners or contractors employed by NSW National Parks and Wildlife Service. We sourced both deer and kangaroo carcasses to ensure enough carrion biomass was collected to start the experiment across all our sites, with both species found within our alpine environment study area. The initial weight, sex and age class of each carcass was recorded alongside the species, wound position, and GPS co-ordinates. Our sites were established at positions that we deemed an appropriate compromise between spatial independence and logistical constraints. The mass mortality plots were placed a minimum of 50 m from each other to minimise potential for interference between treatments while also allowing for the transport and placement of large numbers of carcasses. The single carcass treatments were established a minimum of 100 m from mass mortality plots. We placed each carcass in a lateral recumbent position directly on the ground with the lethal wound facing upward.
Images of example mass mortality plots used in this study. (Top) A combination of fallow deer and eastern grey kangaroo carcasses were used to simulate mass mortalities in our plots. (Bottom) Temporary wire fencing and netting was used to exclude vertebrate scavengers at some mass mortality plots. Images: Stefanie Bonat
Exclusion treatment details. Each of the three carcass treatments was subjected to one of two exclusion treatments: no exclusion (Fig. 1) or with vertebrates excluded (Fig. 1). For the vertebrate exclusion, we constructed wire cages from temporary fencing and netting around carcasses to exclude all vertebrate species including avifauna (e.g., red fox (Vulpes vulpes), wedge tailed eagle (Aquila audax), little eagle (Hieraaetus morphnoides), crow/raven (Corvus spp.), dingo (Canis familiaris dingo), feral pig (Sus scrofa)).
Beetle sampling
We sampled beetles using a pair of pitfall traps in each plot and placed adjacent to a carcass – one trap at the head, the other trap at the back – to collect ground-active insects. The pair of traps at control plots were placed a metre apart. For the mass mortality treatments, we placed a pair of pitfall traps near multiple carcasses to act as insurance against potential interference from vertebrate scavengers. This additional sampling at mass mortality plots meant that we had unbalanced sampling across our treatments, and this dictated our approach to statistical analysis, detailed below. Each pitfall trap was half-filled with polypropylene glycol, an effective killing solution widely used for its low biotoxicity properties (Nakamura et al. 2020). We opened pitfall traps for three days during week one (sample time 1), week two (sample time 2) and week four (sample time 3) of carcass decomposition. These sample times corresponded to contrasting decay ‘stages’ and encompassed fresh > bloat > active (sample 1), active > advanced (sample 2), and advanced > dry > skeletonisation (sample 3) (sensu Payne 1965). Following collection, all adult beetles were removed from traps and sorted into individual species/morphospecies (Oliver and Beattie 1996), counted and identified to family, with some common species also identified to genus and species. Identifications were conducted using relevant keys (Hangay and Zborowski 2010; Lawrence and Slipinski 2013; Matthews 1980, 1982, 1987) and with reference to other carrion beetle studies from south-eastern Australia (Archer 2003; Barton and Evans 2017; Dawson et al. 2022; Evans et al. 2020).
Data analysis
We had more traps placed out for beetles at our mass mortality sites (2–4 pairs of traps) than other sites (one pair of traps only), and we recognised this could bias our data. We therefore created two datasets that were appropriate for answering our questions – (i) a complete but unbalanced dataset, and (ii) a balanced dataset produced by randomly selecting a pair of samples from each mass mortality plot to combine with samples from our other plots. We conducted our analyses of these two datasets as follows: First, we used the complete dataset to examine the taxonomic profile of the beetle community associate with carrion in our study area. To do this we identified the dominant species and families of beetles, and also constructed rank-abundance and rank-richness plots to identify the relative dominance of different beetle families. Second, we used the complete dataset to compare the species richness of beetles found across our carcass treatments. To do this we used species accumulation curves to compare species richness relative to (i) sampling effort and (ii) number individuals collected. We computed observed species richness using the Mau Tau method from our samples using 100 randomisations of the data with the software EstimateS 9.1 (Colwell 2016). Third, we switched to the balanced dataset and used generalised linear mixed models (GLMMs) to test for the effects of carcass treatment, vertebrate exclusion, and sample time on the abundance and species richness of beetles. With this new dataset, however, too few beetles were collected from control sites (no carcass) to include this treatment in a single full model that included all treatment levels and their interactions. Therefore, we took a two-stage approach by first grouping carcass treatments into control vs. carcass, pooling our single and mass mortality data, and ignoring the potential differences between single and mass mortality sites (Model 1). By pooling the carcass treatments we were ignoring potential effects of mass mortality and instead focusing on the magnitude of a general carcass effect relative to the control sites, essentially looking at the ‘hotspot’ effect of carcasses (Barton et al. 2013b; Keenan et al. 2018), and how this changes according to vertebrate exclusion or time. Our fixed effects in this model were carcass addition (carcass vs. control) x vertebrate exclusion (none vs. vertebrate exclusion) x time (weeks 1, 2, or 4). Our second step was to exclude control sites from our dataset and focus on the two carcass types and their interaction with vertebrate exclusion or time (Model 2). Our fixed effects in this model were carcass type (single carcass vs. mass mortality) x exclusion (none vs. vertebrate exclusion) x time (weeks 1, 2, or 4). We used a Poisson error distribution for species richness models and negative binomial distribution for abundance models. We fitted site and sample time as random effects to account for spatial and temporal dependencies in the data. GLMMs were run using GenStat 21 statistical software (VSN International Ltd., 2021). We set our alpha level to < 0.05 to test for significance.
Results
Taxonomic profile of the necrophilous beetle community
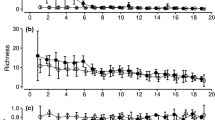
We collected a total of 4,774 beetles representing 146 different species/morphospecies (hereafter referred to as species) from 17 families (see Table S1). In the complete dataset, 89 beetles were sampled from control sites, 1,820 sampled from single carcass sites and 2,865 were sampled from mass mortality sites. Staphylinidae was the most abundant family representing 54.5% of individual beetles, followed by Histeridae (18.9%), Silphidae (5.1%) and Trogidae (4.9%) (Fig. 2a). The major families in terms of species richness were Staphylinidae with 47 species, followed by Scarabaeidae (23 species), Nitidulidae (16 species), and Carabidae (13 species) (Fig. 2b). The most abundant species collected were Saprinus cyaneus cyaneus (711 individuals), an unidentified species of Staphylinidae in the tribe Lomechusini (684), and the congeners Creophilus erythrocephalus (404) and Creophilus lanio (379) (Fig. 2c).
Comparing species richness across carcass treatments
We used species accumulation curves to compare the number of beetle species relative to sampling effort (i.e., species density) and numbers of individuals collected (i.e., species richness) across the carcass treatments. We found that single carcass treatments had the highest number of species relative to sampling effort (approximately 50 samples), followed by mass mortality and then control sites which had the fewest species (Fig. 3a). We then re-expressed numbers of species as a function of the number of individuals collected and found there was little difference between the two carcass treatments (single and mass mortality) the control sites (Fig. 3b), indicating carcasses host a higher density of beetle species but not necessarily a higher richness.
Effects of carcass and exclusion treatments
We first focused on the effects of carcass addition and its interaction with vertebrate exclusion and time. We found a significant interaction between carcass addition and time for both species richness (P = 0.003) and abundance (P < 0.001) of beetles, but no effect of exclusion (Table 1). There was greater beetle abundance at carcasses compared with control sites, and this was greatest during week two of our study (Fig. 4). We next focused on the effect of carcass type (i.e., single vs. mass mortality), and its interaction with vertebrate exclusion and time. We found a significant effect of carcass type on species richness (P = 0.005) and abundance (P = 0.007) and sampling time on species richness (P < 0.001) and abundance (P < 0.001), but no effects of exclusion or any interactions (Table 1). Notably, abundance and species richness was higher at single carcasses than mass mortality carcass, and during week 2 of the experiment (Fig. 5).
Discussion
In this study we aimed to first characterise the necrophilous beetle community found in the study area, and to then determine if beetle assemblages differed between single carcasses, simulated mass mortality carcasses, and carcasses with vertebrates excluded. We found that the beetle fauna included families and key species typically found at carrion resources in other locations in south-eastern Australia. We also detected some unexpected differences between single and mass mortality carcasses, no effect of vertebrate exclusion, as well as a temporal change in the beetle fauna as carcasses decayed. Below we discuss these findings in more detail and draw together some key implications for insect conservation.
The necrophilous beetle fauna
We identified several similarities between our beetle assemblages and those reported from other locations in southeast Australia. The families Staphylinidae and Histeridae were the two most abundant beetle families found at the single and mass mortality sites, and these families are commonly reported from other carrion studies. For example, Barton and Evans (2017), reported high abundances of Staphylinidae and Histeridae, particularly S. cyaneus, on rabbit carcasses in Canberra. In their study, predatory beetles were generally more abundant at carcasses than saprophagous species from the families Dermestidae, Trogidae, or Silphidae, which was also the case in this study. There are also similarities between this study and beetle species reported by Dawson et al. (2021) occurring on pigs and human cadavers near Sydney, notably C. lanio and C. erthycrocephalus, as well as S. cyaneus and Ptomaphila lacrymosa. Further, predatory beetle species were again reported to be more abundant than saprophagous species (Dawson et al. 2021). We also note that there was almost equal abundance of the two congenerics C. erythrocephalus and C. lanio in our study. These two species appear to be morphologically and ecologically similar yet co-occur widely in southern and eastern Australia (Atlas of Living Australia 2022). The study by Dawson et al. (2022) found that C. erythrocephalus was more abundant than C. lanio, but displayed a later peak in abundance relative to C. lanio. More detailed analysis of individual species of beetle is needed to fully explore co-occurrence patterns among species of Creophilus in the study area.
Some beetle species found within our study could be distinct to the study area. An unidentified Staphylinidae in the tribe Lomechusini has not been reported from other studies of carrion insects in Australia yet was the most abundant staphylinid beetle in our study area. Some of the species of dung beetles (Scarabaeidae) we collected may also be unique to the area due to their ability to adapt to differing environmental conditions (Heddle et al. 2021). Dung beetles show variation in their behaviour and distribution in response to abiotic factors and temperature and species occurring in the alpine environment of our study area are likely restricted to cooler temperatures (Heddle et al. 2021).
There were several unexpected beetle species not usually associated with carrion found in our study, including some herbivorous groups. Some species visit the carcass to feed, but others will also be attracted to the carrion in search of prey, hosts or even habitat (Carter et al. 2007). The stomach contents of large herbivores likely attract a range of herbivorous beetles, and this may explain the occurrence of species of Curculionidae, for example (Barry et al. 2019).
Carcass hotspot effects
Relative to control sites, beetles sampled from carcasses were several times more abundant and species rich. This is typical for carrion insects, where carcasses provide a focal point for beetles (and many other insects) to congregate and feed and breed on the rich resources available (Barton et al. 2013b). We found, through species accumulation curves, that single carcass treatments had the highest number of species in relation to sampling effort, followed by mass mortality and then control sites, which had the fewest species. We also found that species richness and abundance was significantly greater at carcass sites than control sites regardless of the vertebrate exclusion treatment. It has been widely demonstrated that carrion generates biodiversity hotspots (Carter et al. 2007; Barton et al. 2013a). This phenomenon can be explained by the cadaver decomposition island (CDI) concept (sensu Carter et al. 2007), and the concentrated biological, chemical and microbial activity occurring in and around a carcass during the decomposition process. CDIs create a specialist habitat for a range of insect taxa including beetles, as demonstrated in this study and elsewhere (Barton et al. 2013b), and play an important role in supporting biodiversity and nutrient recycling, and generating heterogeneity in ecosystems (Barton et al. 2013a; van Klink et al. 2020).
Mass mortality effects
We found that beetles were more abundant at single carcasses than mass mortality carcasses, which was unexpected. We predicted that mass mortality sites would have significantly greater species richness and abundance due to greater resource availability, meaning they could support more individuals and more species. This prediction was based on the theory of island biogeography (MacArthur and Wilson 1967), which states that a larger island will have a greater number of species than a smaller island. Our study did not support this prediction, which raises questions about the analogy of carcasses as mini islands, the appropriateness of our sampling, and most importantly if our contrast between a single versus mass mortality treatments was large enough.
Although there is a rising concern for increasing likelihood of animal mass mortality events (Barton et al. 2022; Fey et al. 2015b), such occurrences are rare and unpredictable unlike naturally occurring resource pulses (e.g., carrion resulting from mass emergence of insects, or large breeding or migration events). One implication of the rarity of mass mortality events is that decomposer populations may not be equipped to consume and process the sudden and large influxes of carrion (Tomberlin et al. 2017). There are very few studies of the effect of differing carrion biomass on arthropod communities. Those studies that do document the effect of carcass size on beetle communities typically focus on small carcasses with little variation among them. For example, a study by Schoenly and Reid (1983) investigated rat carcasses through to rabbit, ranging in size from 14 g to 2.5 kg. This contrasts with our study that used single deer carcasses (average 42 kg body mass) and mass mortality loads consisting of 10 carcasses approximating 1,000 kg in biomass.
We found there was a greater abundance at single carcass sites than that of mass mortality sites. A number of papers have speculated that carrion is likely to be dominated by invertebrate communities at smaller biomasses as opposed to vertebrates (Tomberlin et al. 2017), though there has been no previous work to investigate the effect of large carrion quantities resulting from mass mortality on beetle assemblages, until now. One potential reason for the lower abundance and species richness of beetles at mass mortality carcass sites might be due to the way we sampled beetles. We placed carcasses at mass mortality sites in an area of 8 m x 8 m whereas single carcass sites were only 1.2 m x 1 m, yet the same sampling effort was applied to both. We hypothesise, therefore, that mass mortality and single carcasses likely attracted similar numbers of beetles from the surrounding area, but beetles were potentially more concentrated on single carcass sites due to there being one focal carrion resource only. At the mass mortality sites, there were 10 carcasses for the beetles to colonise which could result in the beetles being diluted among a greater number of carcasses. The net result, therefore, was greater beetle activity around the pitfall traps at single carcasses, and therefore greater captures.
A key finding was the absence of any vertebrate exclusion effect on beetles. Our prediction was that vertebrates would speed up the decomposition of carrion (Devault et al. 2003), and their absence would lead to higher number of beetles due to greater resource availability, particularly at mass mortality sites. Yet we did not find this was the case, and this suggests that vertebrates may not play a critical role in the consumption of large amounts of carrion in mountainous regions of southeast Australia. Further research needs to be conducted to identify the relative contributions of insect and vertebrate scavengers to carrion consumption, and therefore to broader ecosystem function. (Newsome et al. 2021).
A question that stems from our findings involves the role of odours in attracting insects from the surrounding environment. We expected that larger carrion resources would produce greater quantities of odour, although this has not been tested. A result of this might be that larger volumes of odours reach further across the landscape and attract greater numbers of insects. We did not find more beetles at mass mortality sites, although it remains unclear how flies responded. Another role for odours comes from research on volatiles and burying beetles (Coleoptera: Silphidae) which found that beetles might be picky in terms of fresh or deeply decomposed carrion (Trumbo and Steiger 2020). A volatile cue can indicate if a resource is likely too large, too maggot-infested, or too damaged for use by a burying beetle and can operate as a repellent, saving the beetle the energy expense of looking for an unusable resource. Further research is needed to investigate how odour might mediate scavenger attraction and decay rates, or potentially be a deterrent for some species, and how carrion quantity could shape this relationship.
Conclusion and implications
Carrion contributes to landscape heterogeneity and creates a specialist habitat for a number of insects and other scavenger species that enhances landscape biodiversity and drives localised nutrient recycling (Carter et al. 2007; Barton et al. 2013a). Our study found that beetle abundance and species richness was greater at single carcass than at mass mortality sites, and much higher than at controls. Beetle abundance also peaked during active decay, indicating a 2–3 week window of intense insect activity at carcasses. Such findings should be taken into consideration when planning large scale culling programs or responding to mass mortalities resulting from natural disasters. There is a flow-on effect of carrion and intensified culling practices that create animal mass mortality events that scavenging communities may not be adapted too. Understanding how ecological interactions react to an increase of carrion biomass will be critical to comprehending and responding to those consequences (Baruzzi et al. 2018). And as such, how we response or react to mass mortality events can be tailored in areas of environmental significance. If the influx of carrion is human induced, such as the result of a large-scale culling program, such regimes should be planned and timed to reduce environmental impact. Culling programs should be tailored in a way that does not see a large concentration of carcasses in one area, and to mitigate possible carcass pileups, smaller, more frequent culls might be needed. Our data suggests that carcasses might be best colonised by insects if distributed singly. This will focus their densities in a smaller area and potentially remove the carrion more rapidly. Although further research and replication is needed, this study has helped begin addressing some key knowledge gaps outlined by Tomberlin et al. (2017), such as how do mass mortality events affect decomposers and if decomposer communities can functionally respond to the scale of mass mortality events. Further experimental research focusing on yet larger mass mortalities is needed to address this latter question.
References
Archer MS (2003) Annual variation in arrival and departure times of carrion insects at carcasses: implications for succession studies in forensic entomology. Australian J Zool 51:569–576. https://doi.org/10.1071/zo03053
Atlas of Living Australia (2022) www.ala.org.au [accessed: Oct 2022], Vol. 2022
Barry JM, Elbroch LM, Aiello-Lammens ME, Sarno RJ, Seelye L, Kusler A, Quigley HB, Grigione MM (2019) Pumas as ecosystem engineers: ungulate carcasses support beetle assemblages in the Greater Yellowstone ecosystem. Oecologia 189:577–586. https://doi.org/10.1007/s00442-018-4315-z
Barton PS, Evans MJ (2017) Insect biodiversity meets ecosystem function: differential effects of habitat and insects on carrion decomposition. Ecol Entomol 42:364–374. https://doi.org/10.1111/een.12395
Barton PS, Cunningham SA, Lindenmayer DB, Manning AD (2013a) The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 171:761–772. https://doi.org/10.1007/s00442-012-2460-3
Barton PS, Cunningham SA, Macdonald BCT, Mcintyre S, Lindenmayer DB, Manning AD (2013b) Species traits predict Assemblage Dynamics at Ephemeral Resource Patches created by Carrion. PLoS ONE 8:e53961. https://doi.org/10.1371/journal.pone.0053961
Barton PS, Evans MJ, Foster CN, Pechal JL, Bump JK, Quaggiotto MM, Benbow ME (2019) Towards quantifying carrion biomass in ecosystems. Trends Ecol Evol 34:950–961. https://doi.org/10.1016/j.tree.2019.06.001
Barton PS, Reboldi A, Bonat S, Mateo-Tomás P, Newsome TM (2022) Climate-driven animal mass mortality events. is there a role for scavengers? Environmental Conservation in press
Baruzzi C, Mason D, Barton B, Lashley M (2018) Effects of increasing carrion biomass on food webs. Food Webs 17:e00096. https://doi.org/10.1016/j.fooweb.2018.e00096
Baruzzi C, Barton BT, Cove MV, Lashley MA (2022) Mass mortality events and declining obligate scavengers in the Anthropocene: Social feeders may be critical. Biol Conserv 269:109527. https://doi.org/10.1016/j.biocon.2022.109527
Benbow ME, Barton PS, Ulyshen MD, Beasley JC, DeVault TL, Strickland MS, Tomberlin JK, Jordan HR, Pechal JL (2019) Necrobiome framework for bridging decomposition ecology of autotrophically- and heterotrophically-derived organic matter. Ecol Monogr 89:e01331
Bureau of Meteorology (2021) Kosciuszko National Park Weather [Online]. http://www.bom.gov.au/places/nsw/M7VQ/. Accessed 21 July 2021
Carter D, Yellowlees D, Tibbett M (2007) Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 94:12–24. https://doi.org/10.1007/s00114-006-0159-1
Cherubin RC, Venn SE, Driscoll DA, Doherty TS, Ritchie EG (2019) Feral horse impacts on threatened plants and animals in sub-alpine and montane environments in Victoria, Australia. Ecol Manage Restor 20:47–56. https://doi.org/10.1111/emr.12352
Colwell RK (2016) EstimateS 9.1: Statistical estimation of species richness and shared species from samples. (http://purl.oclc.org/estimates)
Dawson BM, Wallman JF, Evans MJ, Barton PS (2021) Is resource change a useful predictor of Carrion Insect Succession on Pigs and humans? J Med Entomol 58:2228–2235. https://doi.org/10.1093/jme/tjab072
Dawson BM, Wallman JF, Evans MJ, Barton PS (2022) Insect abundance patterns on vertebrate remains reveal carrion resource quality variation. Oecologia 198:1043–1056. https://doi.org/10.1007/s00442-022-05145-4
Devault TL, Rhodes JOE, Shivik JA (2003) Scavenging by vertebrates: behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 102:225–234. https://doi.org/10.1034/j.1600-0706.2003.12378.x
Doherty M, Wright G, McDougall K (2015) The flora of Kosciuszko National Park, New South Wales: Summary and overview. Cunninghamia 15:13–68. https://doi.org/10.7751/cunninghamia.2015.15.002
Driscoll DA, Worboys GL, Allan H, Banks SC, Beeton NJ, Cherubin RC, Doherty TS, Finlayson CM, Green K, Hartley R, Hope G, Johnson CN, Lintermans M, Mackey B, Paull DJ, Pittock J, Porfirio LL, Ritchie EG, Sato CF, Scheele BC, Slattery DA, Venn S, Watson D, Watson M, Williams RM (2019) Impacts of feral horses in the australian Alps and evidence-based solutions. Ecol Manage Restor 20:63–72. https://doi.org/10.1111/emr.12357
Endo Y, Nash M, Hoffmann AA, Slatyer R, Miller AD (2015) Comparative phylogeography of alpine invertebrates indicates deep lineage diversification and historical refugia in the australian Alps. J Biogeogr 42:89–102. https://doi.org/10.1111/jbi.12387
Evans MJ, Wallman JF, Barton PS (2020) Traits reveal ecological strategies driving carrion insect community assembly. Ecol Entomol 45:966–977. https://doi.org/10.1111/een.12869
Fey SB, Siepielski AM, Nussle S, Cervantes-Yoshida K, Hwan JL, Huber ER, Fey MJ, Catenazzi A, Carlson SM (2015a) Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proc Natl Acad Sci USA 112:1083–1088. https://doi.org/10.1073/pnas.1414894112
Fey SB, Siepielski AM, Nusslé S, Cervantes-Yoshida K, Hwan JL, Huber ER, Fey MJ, Catenazzi A, Carlson SM (2015b) Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proc Natl Acad Sci USA 112:1083. https://doi.org/10.1073/pnas.1414894112
Green K (2002) Biodiversity in the Snowy mountains / edited by Ken Green. Australian Institute of Alpine Studies, Jindabyne, NSW
Hangay G, Zborowski P (2010) A guide to the Beetles of Australia. CSIRO publishing
Happold DCD (2011) Reproduction and ontogeny of Mastacomys fuscus (Rodentia: Muridae) in the australian Alps and comparisons with other small mammals living in alpine communities. Mammalian Biology 76:540–548. https://doi.org/10.1016/j.mambio.2010.11.006
Heddle TC, Nash M, Henry K (2021) Indigenous and introduced dung beetles (Coleoptera: scarabaeidae) of temperate Australia: a review of biology, importance and effect of climate change on population distributions. Gen Appl Entomol 49:1–11
Keenan SW, Schaeffer SM, Jin VL, DeBruyn JM (2018) Mortality hotspots: nitrogen cycling in forest soils during vertebrate decomposition. Soil Biol Biochem 121:165–176
Lashley MA, Jordan HR, Tomberlin JK, Barton BT (2018) Indirect effects of larval dispersal following mass mortality events. Ecology 99:491–493. https://doi.org/10.1002/ecy.2027
Lawrence J, Slipinski A (2013) Australian beetles volume 1: morphology, classification and Keys. CSIRO Publishing
MacArthur RH, Wilson EO (1967) The theory of Island Biogeography. Princetown University Press, New Jersey
Matthews EG (1980) A guide to the Genera of Beetles of South Australia. Part 1 Archostemata and Adephaga. South Australian Museum, Adelaide
Matthews EG (1982) A guide to the Genera of Beetles of South Australia. Part 2 Polyphaga. Staphylinoidea and Hydrophiloidea. South Australian Museum, Adelaide
Matthews EG (1987) A guide to the Genera of Beetles of South Australia. Part 5 Polyphaga. Tenebrionoidea. South Australian Museum, Adelaide
Mitchell M, Lockwood M, Moore SA, Clement S (2015) Scenario analysis for biodiversity conservation: a social–ecological system approach in the australian Alps. J Environ Manage 150:69–80. https://doi.org/10.1016/j.jenvman.2014.11.013
Nakamura S, Tamura S, Taki H, Shoda-Kagaya E (2020) Propylene glycol: a promising preservative for insects, comparable to ethanol, from trapping to DNA analysis. Entomol Exp Appl 168:158–165. https://doi.org/10.1111/eea.12876
Newsome TM, Barton B, Buck JC, DeBruyn J, Spencer E, Ripple WJ, Barton PS (2021) Monitoring the dead as an ecosystem indicator. Ecol Evol 11:5844–5856. https://doi.org/10.1002/ece3.7542
Olea PP, Mateo-Tomas P, Sanchez-Zapata JA (eds) (2019) Carrion ecology and management, 1st edn. Springer
Oliver I, Beattie AJ (1996) Invertebrate morphospecies as surrogates for species: a case study. Conserv Biol 10:99–109
Payne JA (1965) A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 46:592–602. https://doi.org/10.2307/1934999
Pickering C (ed) (2007) Climate change and other threats in the Australian Alps. In, Protected Areas: buffering nature against climate change. Proceedings of a WWF and IUCN World Commission on Protected Areas symposium, Canberra
Schoenly K, Reid W (1983) Community structure of carrion arthropods in the Chihuahuan Desert. J Arid Environ 6:253–263. https://doi.org/10.1016/S0140-1963(18)31510-6
Tomberlin JK, Barton BT, Lashley MA, Jordan HR (2017) Mass mortality events and the role of necrophagous invertebrates. Curr Opin Insect Sci 23:7–12. https://doi.org/10.1016/j.cois.2017.06.006
Trumbo ST, Steiger S (2020) Finding a fresh carcass: bacterially derived volatiles and burying beetle search success. Chemoecology 30:287–296. https://doi.org/10.1007/s00049-020-00318-0
van Klink R, Van Laar-Wiersma J, Vorst O, Smit C (2020) Rewilding with large herbivores: positive direct and delayed effects of carrion on plant and arthropod communities. PLoS ONE 15. https://doi.org/10.1371/journal.pone.0226946
VSN International Ltd (2021) GenStat for Windows 21st Edition. VSN International Ltd., Hemel Hempstead
Worboys GL, Good RB, Spate A (2011) Caring for our australian Alps catchments: a climate change action strategy for the australian Alps to conserve the natural condition of the catchments and to help minimise threats to high quality water yields, australian Alps. Department of Climate Change and Energy Efficiency, Canberra
Zhu Q, Yang X, Ji F, Liu DL, Yu Q (2020) Extreme rainfall, rainfall erosivity, and hillslope erosion in Australian Alpine region and their future changes. Int J Climatol 40(2):1213–1227. https://doi.org/10.1002/joc.6266
Acknowledgements
We thank Dr Blake Dawson and Anna Reboldi for their valuable assistance with sorting our insect samples. We acknowledge funding from the Hermon Slade Foundation, the Holsworth Wildlife Endowment from the Ecological Society of Australia, and support from the Australian government Research Training Program scholarship. We also would like to thank NSW National Parks and Wildlife service for their ongoing support, and local land managers, who were integral to the success of this experiment, in particular Ted Rowley and Jo Oddie, Michael and Kate Barry, and Ian and Bev Wallace.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Author contributions: TMN, PSB and SB developed the concept of the study and the methodology. SB and TMN did the field work. RLS did the lab work, data curation, analysis. RLS and PSB wrote the first draft. All authors edited the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
The authors report no potential conflict of interest. Scientific licenses and collection permits were obtained to relocate carcasses (SL102334) and research was approved by the University of Sydney Animal Ethics Committee (Project number: 2019/1640).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stone, R.L., Bonat, S., Newsome, T.M. et al. Responses of necrophilous beetles to animal mass mortality in the Australian Alps. J Insect Conserv 27, 865–877 (2023). https://doi.org/10.1007/s10841-023-00504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00504-9