Abstract
The survival of butterfly populations depends on successful oviposition strategies. The limited mobility of early life stages requires females to select sites that reflect larval requirements. However, as land use and climate changes are altering habitat conditions and micro-climate, some species may adapt ovipositing strategies and flourish while others, with narrow niche requirements, may be unable to respond. Oviposition site selection and micro-habitat niche is examined for two closely related butterfly species—the specialist High Brown Fritillary (Fabriciana adippe) and relative generalist Dark Green Fritillary (Speyeria aglaja) through field observations of egg-laying females and analysis of micro-habitat characteristics. A total of 104 oviposition behaviour observations across both species were recorded in 69 1 m2 quadrats, with the habitat characteristics compared to randomly selected quadrats in the same area. Results show that higher host plant density was a positively significant factor for oviposition site selection only for the High Brown Fritillary. Moreover, the cover of live Bracken (Pteridium aquilinum) and grass were important for site selection in both species, with High Brown Fritillaries tolerating less live Bracken and grass cover than Dark Green Fritillaries. This confirms the more specific requirements and narrower micro-habitat niche of the High Brown Fritillary, which appears to be more sensitive to micro-habitat cooling.
Implications for insect conservation
The management of Bracken mosaic habitats for these two species should aim to supress grass growth and maintain Bracken density within limits, by opening the Bracken canopy on a rotation through grazing or manual cutting, ensuring a continuous supply of suitable micro-habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the extreme global declines in invertebrates (Wagner et al. 2021), Lepidoptera are one of the most affected taxa (Sánchez-Bayo and Wyckhuys 2019) with declines reported across Europe (Maes and Van Dyck 2001; van Swaay et al. 2010; Stefanescu et al. 2011; Warren et al. 2021), North America (Forister et al. 2011; Wepprich et al. 2019) and Asia (Nakamura 2011; Choi and Kim 2012). Butterfly declines are attributed to agricultural intensification, abandonment and fragmentation at the landscape level (van Swaay et al. 2010) along with direct habitat loss, changes in management practices and habitat quality at the local level. Within this order, it is the habitat specialists that have disproportionately declined (van Swaay et al. 2006; Stefanescu et al. 2011) and are more at risk from extinction on a local level (Ellis et al. 2012). An understanding of the autecology of all life-stages is required for successful conservation of lepidoptera, particularly since most species typically spend much of their life cycle in the juvenile stages. Indeed, the requirements of eggs and larvae can be more specific than that of adults (Smee et al. 2011), highlighting the greater need to define habitat quality for these immature stages prior to habitat management (Thomas et al. 2011).
The limited mobility of early life-stages implies that successful oviposition strategies are critical for the survival of butterfly populations (García-Barros and Fartmann 2009). These strategies involve complex processes to select sites with optimal conditions that promote the development of the offspring. This site selection occurs at the landscape, patch, and micro-habitat scale and can be influenced by human induced environmental change (García-Barros and Fartmann 2009).
A butterfly will detect a suitable site for oviposition through chemical, visual and thermal cues, assessing the habitat, micro-habitat and prospective host plant (Singer 2004; García-Barros and Fartmann 2009; Eilers et al. 2013). The most common strategy is to lay eggs directly on the larval food plant, enabling emerging larvae to feed without depleting their energy reserves (Wiklund 1984). However, species which overwinter in the egg stage and whose larvae feed on herbaceous plants which die out in winter deviate from this approach. Such species avoid laying on the host plant, choosing instead to lay on a sturdy substrate nearby (Wiklund 1984; Barnett and Warren 1995; Kopper et al. 2000).
Such a life-history strategy, with overwintering eggs laid on nearby substrate, is rare with few species recorded in the literature. The only UK butterfly to adopt this strategy is the High Brown Fritillary (Fabriciana adippe, Dennis & Schiffermüller, 1775). It is a habitat specialist occupying sheltered south-facing Bracken [Pteridium aquilinum (L.) Kuhn] dominated slopes interspersed with grassy areas (Barnett and Warren 1995). Egg laying occurs in gaps in the Bracken canopy, where Bracken litter is dominant and grass is sparse (Warren 1995a). In NW England it also utilises early successional habitat in woodland clearings and rides, where egg laying occurs in short vegetation near rock outcrops, with a good cover of moss and sparse grass. These micro-habitats are warmer than ambient temperature and surrounding vegetation on sunny days (Warren 1995a).
The larvae of the High Brown Fritillary hatch in March to feed on the primary larval food plant, Common Dog violet (Viola riviniana Rchb.), however Hairy violet (Viola hirta L.) is also used in NW England (Warren et al. 1995). To aid development, larvae bask on moss or Bracken litter which can be 15–20 °C warmer than the surrounding short grassy vegetation (Asher et al. 2001). Previous studies have observed larvae in similar habitats to those selected for oviposition (Warren 1995a). The visually similar Dark Green Fritillary (Speyeria aglaja, Linnaeus, 1758) inhabits a broadly similar habitat although is a relative generalist occupying a wider range of habitats (Polic et al. 2021). The Dark Green Fritillary overwinters as larvae and tolerates a cooler vegetation for breeding (Asher et al. 2001; Ellis et al. n.d.). It also utilises Viola spp. as the primary larval host plant in Bracken habitats, although is also known to feed on Bistort [Persicaria bistorta (L.) Samp.] in humid grasslands (Fric et al. 2005; Zimmermann et al. 2009).
The Dark Green Fritillary is not of conservation concern in Europe due to its stable range but is Near Threatened in the UK due to reductions in distribution with significant range contractions over the last 10 years (Fox et al. 2022). In contrast, the High Brown Fritillary has suffered declines across Europe, most markedly in the UK, with a severe range contraction [− 82% since 1985 (Fox et al. 2023)] and decrease in abundance [− 65% since 1978 (Fox et al. 2023)]. As a result, it is the UK’s fastest declining butterfly (Ellis et al. 2015), is recognised as Endangered on the Red List of British butterflies (Fox et al. 2022) and is protected in UK legislation [Natural Environment and Rural Communities Act (2006); Environment (Wales) Act (2016); Wildlife & Countryside Act (1981)].
Research into these two species typically focuses on dispersal and colonisation (Six 2000; Cowley et al. 2001a, 2001b; Zimmermann et al. 2009; Nesbitt 2010; Polic et al. 2021). The difficulties of direct observation of oviposition for these species has resulted in only a few published accounts (Thomas 1991; Zimmermann et al. 2009) and limited research into micro-habitat selection (Warren 1995a, 1995b; observations n = 22, n = 27 respectively). The females of both species are strong fliers and can rapidly drop into dense Bracken stands making them difficult to observe. Female High Brown Fritillaries will alight on host plants and non-host plants alike to detect suitable oviposition sites (Wiklund 1984). Egg laying occurs on sturdy substrate most often after crawling over the leaf of the nearby host plant, although occasionally eggs are laid soon after landing, before encountering any Viola spp. (Barnett and Warren 1995; Warren 1995a).
Studying the habitat quality at the site of oviposition in two similar species within the same habitat will provide a new understanding of micro-habitat requirements and niche separation. We predict that oviposition sites of both species will have higher density of host plants than the surrounding habitat. Based on the acknowledged differences in larval thermal preferences, it is expected that High Brown Fritillary females will choose oviposition micro-habitats with greater bracken litter cover and lower cover of grass that will aid micro-climatic warming. In contrast, the relatively generalist Dark Green Fritillaries will be less specific in choosing oviposition sites using a broader range of micro-habitats.
Methodology
Study area
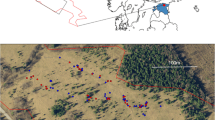
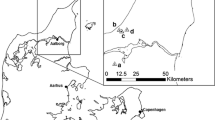
Data was collected within the Morecambe Bay area (54°12′07″N, 002°42′07″W), a lowland landscape in NW England characterised by farmland, limestone grasslands, broadleaved woodland and exposed limestone pavements (Natural England 2022). This area accounts for most of the UK High Brown Fritillary colonies (Jones et al. 2015) and the Dark Green Fritillary is also present.
The colony inhabiting the adjacent management units of Holme Stinted Pastures and the south-western corner of Holme Park Fell was chosen for this study due to its medium population size and recently stable numbers of High Brown Fritillary (Ellis and Wainwright 2008). The area is approximately 20 ha, with an elevation of 130–170 m a.s.l and is predominately south-facing. The principal habitat is Bracken and limestone grassland mosaic with scrub (Online Resource, Figure S1). Prior to 2000, Holme Park Fell was heavily grazed by sheep and now has reduced stocking densities of cattle and sheep, whereas Holme Stinted Pastures had historically low stocking densities, with a scrub management programme introduced since 2008 (Ellis and Wainwright 2008). Both management units are now in agri-environment schemes, are grazed by cattle, and a programme of scrub removal and light Bracken control aimed at increasing breeding habitat has been implemented in recent years.
Oviposition observation
Due to habitat variability across the site, the study area was divided into thirteen sections of potentially suitable habitat. Each section was surveyed for male and female adults by walking a set route on days with dry weather above 13 °C from the beginning of the short flight period of both species. The entire route through all sections was walked 13 times over 10 days between 11th June & 14th August in 2020, and 22 times over 14 days between 12th June & 4th August in 2021, after which sections with the most sightings of adults were targeted to maximise the chance of observing oviposition behaviour. Species ID was carried out in the field using binoculars & photography to determine species and sex.
After locating a female of either species, they were followed from a distance of at least 3 m for up to 30 min, until they settled or were lost from sight. Upon settling they were approached to 1 m (Kopper et al. 2000) and observed for oviposition behaviour. Oviposition behaviour was determined as an observed and definite downward turn of the abdomen accompanied by probing of the substrate. Oviposition behaviour sites were waymarked using a handheld GPS device and a coloured peg was placed as close to the location as possible without causing disturbance (Online Resource, Figure S2). Eggs were searched for and only found occasionally. Due to this lack of verification of oviposition the marked sites represented oviposition behaviour. If an individual showed no oviposition behaviour within the 30 min further females would be searched for in the same site section (Henry and Schultz 2013). If both species were observed at the same time, preference was given to following High Brown Fritillaries due to the conservation status of this species.
To further verify oviposition success, larval feeding damage to the host plant was recorded the following year on 11th June 2021 & 7th June 2022 in each of the observed oviposition behaviour sites. Larvae were considered present if there were any Viola spp. plants with heavy damage (> 30% of a leaf) or more than 4 plants with medium damage (11–30% of a leaf) within the quadrat. Due to the presence of several Viola-feeding fritillary species on site, the species causing the damage could not be determined. Larval searches were carried out, but observations were low (n = 1), therefore, larval performance could not be accurately assessed. No significant difference (P > 0.05) was found in the habitat between oviposition behaviour sites with confirmed eggs or larval damage and those without (Online Resource, Table S3 & 4). This suggests that in the absence of oviposition verification, the observation of oviposition behaviour is an appropriate method for determining site selection of ovipositing females for these species.
Micro-habitat data collection
Within two weeks of the observation a 1 m2 quadrat was placed centrally around each point of oviposition behaviour, where the edges were perpendicular to cardinal directions. Oviposition behaviour by the same individual that fell within 30 cm of each other were recorded within the same quadrat to minimise overlap of quadrats.
To determine differences in micro-habitat preferences between both species, a range of variables which affect the micro-habitat temperatures through shading and insulation, or the ability of the host plant to develop through competition and resource limitation, were measured in each quadrat (Online Resource, Table S5). These were leaf litter depth and percentage cover of Bracken, Bracken litter, grass, moss, bare ground and rock, and Bramble. To determine the influence of host plant density, the number of Viola spp. per m2 and the distance from the point of oviposition behaviour to the nearest Viola spp. plant (mm) were measured. In quadrats with more than one oviposition behaviour observation, measurements taken at the site of oviposition behaviour were averaged to gain one reading.
To determine micro-habitat preference within each species, randomly generated locations of the wider available habitat in each section of the site were surveyed as controls. Randomly generated locations were rejected for survey if they were in areas of dense trees or scrub over 2 m as this did not represent the habitat utilised by either species. To avoid surveyor bias and for ease of resurveying, the 1m2 quadrat was placed to the north-east of each randomly generated control location with the edges perpendicular to cardinal directions. Control locations were surveyed for the same variables within four weeks of the first oviposition behaviour in each section. This led to a total of 97 control locations, reflecting the number of oviposition behaviour observations in each section as well as 28 locations in an additional section where fritillaries had been observed regularly but no oviposition behaviour recorded.
Data analysis
Analyses were carried out with R software, version 4.2.1 (R Core Team 2022).
To determine if the butterflies select distinct micro-habitats for oviposition, Principal Component Analyses was used to compare the microhabitat variables at oviposition behaviour sites with those at control locations. One PCA was carried out for each species using the RDA function in the ‘vegan’ package (Oksanen et al. 2022) and the variables were standardised by mean zero and unit variance.
To test if oviposition behaviour is determined by host plant density and other micro-habitat variables, a binary generalized linear model (GLM) with model averaging was carried out for each species. Prior to modelling, co-linearity between variables was checked using the variance inflation factor (VIF) function within the ‘car’ package (Fox and Weisberg 2019). Variables with the highest VIF were sequentially dropped until all remaining variables had a VIF value < 3 (Zuur et al. 2010) (Online Resource, Tables S6 & S7). The rcorr function within the ‘Hmisc’ package (Harrell Jr 2022) was also used to calculate the correlation coefficient (rho) between response variables for each species (Online Resource, Figures S8 & S9). Any remaining variables with a high correlation (rho > ± 0.7) were assessed for inclusion in the models and the least important variable in terms of biological rationale was removed. The final variables selected for modelling in both species were Viola spp. density, average litter depth, cover of Bracken, grass, moss, bare ground and rock, and Bramble (Rubus fruticosus agg.) (Online Resource, Table S5). Quadratic terms were included for cover of Bracken and grass for both species and Bramble for Dark Green Fritillary which displayed non-linear relationships. A binomial error distribution was applied to each model with binary response variables (0 = control, 1 = oviposition behaviour observation in each species respectively). Three global models were produced for each species to enable the selection of the most appropriate link function with the lowest residual deviance. ‘Probit’ link function was used for the models of both species.
Multi-model inference with the ‘MuMin’ package (Barton 2022) was used to assess all possible models for each species as the large combination of variables produced too many models to assess manually. Models were standardised for by mean zero and unit variance. The model with the best fit was determined by the lowest AICc. All models within AICc ≤ 2 of the best model for each species were checked prior to model averaging with the Hosmer–Lemeshow goodness of fit test using the Hoslem.test function in the ‘ResourceSelection’ package (Lele et al. 2019). All models showed no significant difference between the model and the observed data for each species (P > 0.05) and thus represented a good fit. The final ‘best’ model for each species was validated by replacing correlated variables to ensure that the removed variables did not improve the AICc. All variables were assessed for relative variable importance (RVI) based on the sum of the Akaike weights (\({w}_{i}\)) from all possible models for each species (Burnham and Anderson 2002). The inclusion of quadratic terms resulted in each variable appearing in an unequal number of models, therefore RVI was divided by the number of models each variable appeared in to gain an adjusted RVI (Kittle et al. 2008).
To explore niche separation, PCA was used to compare the microhabitat variables at the oviposition behaviour sites of each species, as previously described. To determine if the species select different microhabitats for oviposition, each habitat variable was tested for significant difference using either a Two sample t-test or a non-parametric Wilcoxon rank sum test.
Results
Oviposition observation
Sixty-five High Brown Fritillary and thirty-nine Dark Green Fritillary oviposition behaviours were recorded in 69 1 m2 quadrats across 9 sections of the site (Table 1; see Online Resource for further details). Following searches at each location no eggs were found in 2020 while five Dark Green and two High Brown Fritillary eggs were found in 2021. Two of the Dark Green Fritillary eggs were found within the same quadrat on two separate Viola spp. plants. All quadrats with eggs had heavy larval damage the following spring and a further twelve Dark Green and seventeen High Brown Fritillary oviposition quadrats also had larval damage. 33% of Dark Green Fritillary and 58% of High Brown Fritillary quadrats did not have significant feeding damage or eggs. It could not be confirmed which species or which individual caused the feeding damage and it is possible that unobserved oviposition events by other individuals occurred in the same locations.
High Brown Fritillary oviposition site selection
A PCA of all habitat variables at High Brown Fritillary oviposition behaviour sites and across the control locations accounted for 49% of the variation in this dataset in PC1 (35%) & PC2 (14%) (Fig. 1; Online Resource, Table S10). Control locations were distributed across the entire spread of the oviposition behaviour sites in ordination space and thus are a true representation of the available habitat. High Brown Fritillary oviposition behaviour showed a negative association with PC2, which is negatively associated with cover of bare ground and rock and Viola spp. density and positively associated with cover of Bramble and leaf litter depth. Within the observed oviposition behaviour sites there was no separation of quadrats with confirmed eggs or larval damage and those without.
Binary GLMs revealed that the best model for predicting oviposition site selection in High Brown Fritillaries included cover of Bracken and cover of grass (both quadratic and linear terms) and Viola spp. density. In this model the quadratic term for cover of Bracken and linear term for cover of grass were significant at the P < 0.001 level, whereas Viola spp. density was significant at the P < 0.05 level (Table 2). The adjusted RVI revealed cover of Bracken and grass to be the most important. Multi-model inference revealed three other valid models within AICc ≤ 2 of the best model. Model averaging of these four models showed that cover of Bracken, cover of grass (P < 0.001) and Viola spp. density (P < 0.01) were significant in the selection of oviposition sites by the High Brown Fritillary, with relative variable importance indicating Viola spp. density to be the least important variable of the three. Cover of Bramble and cover of moss were included in the averaged model but were not found to be significant (Table 2).
Visualisation of the best model reveals the non-linear relationship between the explanatory variables of Bracken and grass cover and the probability of oviposition behaviour in the High Brown Fritillary (Fig. 2). The probability of oviposition behaviour increases rapidly with the cover of Bracken, reaching more than 0.8 between 30 and 65% cover of Bracken. Above 65% Bracken cover probability rapidly decreases. The probability of oviposition behaviour reaches more than 0.8 when grass cover is between 15 and 35%. Above 45% grass cover probability rapidly decreases. The presence of Viola spp. gives a nearly 0.8 probability of oviposition behaviour by High Brown Fritillary. As the number of Viola spp. increases to 100 per m2 probability of oviposition behaviour rises to 0.9.
Conditional plots of the relationship between probability of High Brown Fritillary oviposition behaviour and each significant explanatory variable including; a % cover of Bracken (P < 0.001), b % cover of grass (P < 0.001), and c Viola spp. density (P < 0.05), as estimated by the best model (AICc = 87.58)
Dark Green Fritillary oviposition site selection
A PCA of all habitat variables collected at Dark Green Fritillary oviposition sites and across the control locations accounted for 54% of the variation in this dataset in PC1 (39%) & PC2 (15%) (Fig. 3; Online Resource, Table S11). Control locations were distributed across the entire spread of the oviposition behaviour sites in ordination space and thus are a true representation of the available habitat. Dark Green Fritillary oviposition behaviour was negatively associated with PC1, which is negatively associated with cover of grass, Viola spp. density and cover of moss, and positively associated with cover of Bracken, cover of Bracken litter and distance to nearest Viola spp.. Within the observed oviposition behaviour sites there was no separation between quadrats with confirmed eggs or larval damage and those without.
The best model for predicting oviposition site selection in Dark Green Fritillaries included cover of Bracken and cover of grass (both quadratic and linear terms) (Table 3). The quadratic & linear terms for cover of Bracken were significant at the P < 0.001 level, and the linear term for cover of grass was significant at the P < 0.05 level. The adjusted RVI revealed cover of Bracken and grass to be the most important. Multi-model inference revealed six other valid models within AICc ≤ 2 of the best model. Model averaging also showed that quadratic and linear terms cover of Bracken (P < 0.05) and the quadratic term for grass (P < 0.01) were significant in the selection of oviposition sites for the Dark Green Fritillary. Other variables were included in the averaged model but were not significant (Table 3).
Visualisation of the best model reveals the non-linear relationship between the significant explanatory variables and the probability of oviposition behaviour in the Dark Green Fritillary (Fig. 4). The probability of oviposition behaviour reaches more than 0.7 between 25 and 55% cover of Bracken, above which probability rapidly decreases. The probability of oviposition behaviour is greatest when grass cover is between 30 and 60%, above which probability slowly decreases.
Micro-habitat niche-separation
A PCA of all habitat variables collected at High Brown Fritillary and Dark Green Fritillary oviposition behaviour sites accounted for 40% of the variation in this dataset in PC1 (25%) & PC2 (15%) (Fig. 5). Within the observed oviposition behaviour sites there was no separation of sites with confirmed eggs or larval damage for either species, although there was separation of oviposition behaviour sites between the species. The separation was predominantly associated with PC1, most explained by cover of Bracken litter, cover of Bracken, and cover of grass. Dark Green Fritillary oviposition was also positively associated with PC2, most explained by Viola spp. density, cover of grass and cover of Bramble (Online Resource, Table S12).
Assessment of individual habitat variables between oviposition behaviour sites of the two species showed that there was a significant difference in the cover of Bracken (t = − 2.2318; df = 67; P = < 0.05), with High Brown Fritillaries choosing sites with more Bracken than Dark Green Fritillaries (Fig. 6a). Cover of grass differed significantly between the two species’ oviposition behaviour sites (W = 912.5; N = 69; P = < 0.001), with High Brown Fritillaries choosing sites with less grass cover than Dark Green Fritillaries (Fig. 6b). There was a significant difference in the cover of Bracken litter between the two species’ oviposition behaviour sites (W = 264; N = 69; P = < 0.005), with High Brown Fritillaries choosing sites with more Bracken litter than Dark Green Fritillaries (Fig. 6c). Cover of Bramble differed significantly between the two species oviposition behaviour sites (W = 697; N = 69; P = < 0.05), with High Brown Fritillaries choosing sites with less Bramble cover than Dark Green Fritillaries (Fig. 6d). Viola spp. density did not significantly differ (P > 0.05) between the oviposition behaviour sites of the two species (Fig. 6e). Leaf litter depth, cover of moss, and cover of bare ground and rock were also not significantly different (all P-values > 0.05, see Online Resource, Figure S13).
Boxplots of five habitat variables; a cover of Brackena; b cover of grassb; c cover of Bracken litterb; d cover of Bramblea; and e Viola spp. density. Control locations were not included in analysis. DGF = Dark Green Fritillary oviposition behaviour sites (n = 24), HBF = High Brown fritillary oviposition behaviour sites (n = 45), Control = control locations (n = 97). aSignificant difference (P < 0.05) between species. bSignificant difference (P < 0.001) between species
Discussion
Oviposition site selection
Oviposition site selection by High Brown Fritillary was significantly affected by the cover of live Bracken, the cover of grass and the density of Viola spp., whilst oviposition site selection by the Dark Green Fritillary was also significantly influenced by the cover of live Bracken and the cover of grass, but not by density of the host plant. These variables presumably affect the development of larvae through changes in micro-habitat temperature and resource availability and the influence of host plant density for the High Brown Fritillary suggests this species has additional requirements.
Warm micro-habitats with larval host plants promote faster larval development through increased (Rytteri et al. 2021) and more efficient feeding (Porter 1982), and in turn can lower the risk of predation and parasitism (Benrey and Denno 1997). Due to micro-climatic cooling by tall, green vegetation (WallisDeVries 2006; WallisDeVries and van Swaay 2006), it was expected that oviposition behaviour would be significantly negatively influenced by an increase in cover of grass. The results of this study show that, whilst both species tolerate some grass, the chance of oviposition behaviour decreases rapidly beyond a threshold of approximately 45% cover for the High Brown Fritillary and slowly beyond 60% for the Dark Green Fritillary. Similarly, both species selected sites with an intermediate cover of live Bracken (High Brown Fritillary: 35–65%, Dark Green Fritillary: 25–55%), suggesting intermediate coverage is optimal. These findings for the High Brown Fritillary align with Warren (1995a) where, in Bracken habitats in NW England, oviposition was observed in locations with a Bracken canopy between 30 and 70% and grass cover of 12–40%.
Micro-habitat preferences at the time of oviposition may reflect the larval preference for specific micro-climatic conditions. A dense canopy of Bracken is not present in the early spring when larvae are developing thus not likely to influence the performance of larvae, although females using thermal cues to find optimal conditions for their offspring could still be deterred by such cool environments in the summer.
Tolerating some microclimatic cooling during oviposition may be a trade-off as the amount of shade produced by standing Bracken provides a canopy for Viola spp., a species which thrives in woodlands (Warren and Oates 1995; Ellis 2005). Too little Bracken may be associated with a limited production of Bracken litter and increased presence of grass, which will further cool the micro-habitat at oviposition sites. Due to the absence of a Bracken canopy in the spring, when habitat assessments for the two species are normally carried out, the amount of standing live Bracken has not been previously identified as a significant factor in micro-habitat quality for either species.
The ability of Bracken litter to reach and maintain the optimum temperature for larvae (32 °C) on mild days (Warren 1994) and the numerous accounts of larvae found basking in these locations would indicate that it would be influential in oviposition site selection. As such, it was expected that Bracken litter could positively influence site selection but due to the co-variation with standing live Bracken, this was not displayed by the models. The preference for standing live Bracken over Bracken litter may reflect a difference in visual apparency of these two variables to ovipositing females.
Survival for the relatively immobile early instars is reliant on females selecting oviposition sites within reach of a food resource. Host plant density is shown to positively influence occurrence, oviposition and emigration in a range of lepidoptera (Menéndez et al. 2002; Betzholtz et al. 2007; Salz and Fartmann 2009; Smee et al. 2011; Ewing et al. 2020). Viola spp. density was an important factor in site selection only for the High Brown Fritillary, however there was no difference in the density of host plants between the sites selected by the two species. The indication that Viola spp. density is not an important factor in site selection for the Dark Green Fritillary species is unexpected due to the lack of alternative host plants within Bracken habitats.
Viola spp. are not uncommon plants, nor are they restricted to just one habitat. Therefore, the presence of the plants alone is not likely a driving factor in the occurrence of each species. The relative importance of the significant variables indicates that High Brown Fritillary females may prioritise habitat structure and composition as an oviposition cue whilst only secondarily assess host plant density. Such prioritisation has been evidenced in other Lepidopteran species (Friberg et al. 2008b), although this may not be applicable if host plant density is too low.
If faced with an abundance of host plants, other factors of host plant quality may become influential in oviposition site selection, this may include more apparent features including host plant size (Anthes et al. 2003) or colour (Myers 1985; Stefanescu et al. 2006). Less apparent physiological measures, such as levels of carbon, nitrogen and phosphorous (Myers 1985; Salz and Fartmann 2009) and defensive compounds (García-Barros and Fartmann 2009) could also indirectly influence oviposition site selection. Consequently, host plant selectiveness may change over time with agricultural intensification and anthropogenic nitrogen deposition.
Micro-habitat niche-separation
Micro-habitat is of importance for adults of both species when selecting oviposition sites, but our results indicate that the High Brown Fritillary requirements are more specialised (Barnett and Warren 1995a, b). Dark Green Fritillaries chose oviposition sites with a significantly higher cover of grass and a lower cover of Bracken litter and standing live Bracken than High Brown Fritillaries, affirming the higher tolerance of Dark Green Fritillaries to micro-climatic cooling. Whilst a denser Bracken canopy will cool the micro-climate in summer, the amount of litter that it produces in autumn, together with its ability to supress grass growth, is also of importance to the High Brown Fritillary (Warren and Oates 1995).
The wider variety of suitable habitat types available to the Dark Green Fritillary within its range (Zimmermann et al. 2009; Polic et al. 2021) suggests a wider habitat niche than the specialist High Brown Fritillary. The results of this study also confirm significant differences in micro-habitat niche between the two species within their overlapping range. The wider niche of the Dark Green Fritillary could be advantageous for persistence at the landscape scale as the need for long distance dispersal will be reduced when faced with changes in micro-habitat (Six 2000; Polic et al. 2021). However, it is important to take into account that niche separation between two closely related species has been shown to vary with local habitat variation across geographic ranges (Friberg et al. 2008a), and that oviposition preference in High Brown Fritillaries may differ between recently cleared scrub and Bracken habitats (Warren 1995a). As such, oviposition preference could differ in a more ubiquitous habitat, where Bracken is more extensive and recently removed scrub is not a key part of the available habitat.
Conservation implications
Management of Bracken mosaic habitats for the High Brown and Dark Green Fritillary should aim to restore and maintain micro-habitat with a combination of 35- 65% cover of Bracken, less than 45% cover of Grass and a high density of Viola spp. (> 100/m2). These limits should provide suitable breeding habitat for both species to co-exist in the same habitat area, although the extent of suitable micro-habitat may need to be considered at the site and landscape scale to support viable metapopulations.
The seemingly contradictory habitat requirement of less than 65% cover of live Bracken and a good cover of Bracken litter demonstrates the fine line in management of Bracken for the more specialised High Brown Fritillary. The areas on site where this occurred were largely where cattle had created paths through Bracken in the summer months, keeping the Bracken sward open and breaking down the previous year’s litter. Where grazing is not possible, cutting paths through Bracken early in the flight season would open dense swards enabling warming of Bracken litter below. This would also enable regrowth later in the season to supress grass growth and maintain a supply of Bracken litter for the following breeding season. To enable a continuous supply of this habitat, it is likely that the network of paths would need to change year on year as paths remaining open for too long can quickly be invaded by grass. Stocking densities should be low enough for this to happen and cutting will need to be on rotation.
Due to the conservation status and specialised habitat requirements of the High Brown Fritillary, management should be directed towards this species, however the Dark Green Fritillary and other Viola-feeding fritillaries with wider niches (e.g. Pearl-bordered and Small Pearl-bordered Fritillaries) are also likely to benefit (Ellis et al. 2019).
References
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—The key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7(3):175–185. https://doi.org/10.1023/A:1027330422958
Asher J, Warren M, Fox R, Harding P, Jeffcoate G, Jeffcoate S (2001) The Millennium Atlas of Butterflies in Britain and Ireland. Oxford University Press, Oxford
Barnett LK, Warren MS (1995) Species Action Plan High Brown Fritillary Argynnis adippe. Butterfly Conservation, Wareham, Dorset
Barton K (2022) MuMIn: Multi-Model Inference. R Package version 1.46.0. https://cran.r-project.org/package=MuMIn
Benrey B, Denno RF (1997) The slow-growth–high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78(4):987–999. https://doi.org/10.2307/2265852
Betzholtz PE, Ehrig A, Lindeborg M, Dinnétz P (2007) Food plant density, patch isolation and vegetation height determine occurrence in a Swedish metapopulation of the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae). J Insect Conserv 11(4):343–350. https://doi.org/10.1007/s10841-006-9048-3
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edn. Springer
Choi S-W, Kim S-S (2012) The past and current status of endangered butterflies in Korea. Entomol Sci 15:1–12. https://doi.org/10.1111/j.1479-8298.2011.00478.x
Cowley MJR, Thomas CD, Roy DB, Wilson RJ, León-Cortés JL, Gutiérrez D, Bulman CR, Quinn RM, Moss D, Gaston KJ (2001a) Density-distribution relationships in British butterflies. I. The effect of mobility and spatial scale. J Anim Ecol 70(3):410–425. https://doi.org/10.1046/j.1365-2656.2001.00508.x
Cowley MJR, Thomas CD, Wilson RJ, León-Cortés JL, Gutiérrez D, Bulman CR (2001b) Density-distribution relationships in British butterflies. II. An assessment of mechanisms. J Anim Ecol 70(3):426–441. https://doi.org/10.1046/j.1365-2656.2001.00509.x
Eilers S, Pettersson LB, Öckinger E (2013) Micro-climate determines oviposition site selection and abundance in the butterfly Pyrgus armoricanus at its northern range margin. Ecol Entomol 38(2):183–192. https://doi.org/10.1111/een.12008
Ellis S (2005) Bracken monitoring for fritillary butterflies on the Morecambe Bay Limestones. Butterfly Conservation report no. SO 5-0 3. Butterfly Conservation, Wareham, Dorset
Ellis S, Wainwright D (2008) Conservation of the High Brown Fritillary Argynnis adippe and Pearl-bordered Fritillary Boloria euphrosyne butterflies in North West England. Butterfly Conservation Report No. S08–27. Butterfly Conservation, Wareham, Dorset
Ellis S, Bourn N, Bulman C, Hobson R, Jones R, Middlebrook I, Plackett J, Smith R, Wain M, Wainwright D, Wainwright D, Warren M (2015) Conserving Britain’s fastest-declining butterfly. Br Wildl 27(2):111–122
Ellis S, Wainwright D, Dennis EB, Bourn NAD, Bulman CR, Hobson R, Jones R, Middlebrook I, Plackett J, Smith RG, Wain M, Warren MS (2019) Are habitat changes driving the decline of the UK’s most threatened butterfly: the High Brown Fritillary Argynnis adippe (Lepidoptera: Nymphalidae)? J Insect Conserv 23(2):351–367. https://doi.org/10.1007/s10841-019-00134-0
Ellis S, Warren M, Brereton T, Toynton P, Chandler D. (n.d.) Dark Green Fritillary factsheet. https://butterfly-conservation.org/butterflies/dark-green-fritillary. Accessed 23 Feb 2023
Ewing SR, Menéndez R, Schofield L, Bradbury RB (2020) Vegetation composition and structure are important predictors of oviposition site selection in an alpine butterfly, the Mountain Ringlet Erebia epiphron. J Insect Conserv 24(3):445–457. https://doi.org/10.1007/s10841-020-00229-z
Forister ML, Jahner JP, Casner KL, Wilson JS, Shapiro AM (2011) The race is not to the swift: long-term data reveal pervasive declines in California’s low-elevation butterfly fauna. Ecology 92(12):2222–2235. https://doi.org/10.1890/11-0382.1
Fox J, Weisberg S (2019) An R Companion to Applied Regression, 3rd edn. Sage, Thousand Oaks CA
Fox R, Dennis EB, Brown AF, Curson J (2022) A revised Red list of British butterflies. Insect Conserv Divers 15(5):1–11. https://doi.org/10.1111/icad.12582
Fox R, Dennis E, Purdy K, Middlebrook I, Roy D, Noble D, Botham M, Bourn N (2023) The State of UK’s Butterflies 2022. Wareham, UK
Friberg M, Bergman M, Kullberg J, Wahlberg N, Wiklund C (2008a) Niche separation in space and time between two sympatric sister species-a case of ecological pleiotropy. Evol Ecol 22(1):1–18. https://doi.org/10.1007/s10682-007-9155-y
Friberg M, Olofsson M, Berger D, Karlsson B, Wiklund C (2008b) Habitat choice precedes host plant choice—Niche separation in a species pair of a generalist and a specialist butterfly. Oikos 117(9):1337–1344. https://doi.org/10.1111/j.0030-1299.2008.16740.x
Fric ZF, Klimova M, Hula V, Konvička M (2005) Caterpillars of Argynnis aglaja (Linnaeus, 1758) feeding on Bistorta major (Lepidoptera, Nymphalidae). Atalanta 36(1/2):119–121
García-Barros E, Fartmann T (2009) Butterfly oviposition: sites, behaviour and modes. In: Settele J, Shreeve T, Konvička M, van Dick H (eds) Ecology of Butterflies in Europe, 1st edn. Cambridge University Press, Cambridge, pp 29–42
Harrell Jr FE (2022) Hmisc: Harrell miscellaneous. R Package version 4.7-0. https://cran.r-project.org/package=Hmisc
Henry EH, Schultz CB (2013) A first step towards successful conservation: understanding local oviposition site selection of an imperiled butterfly, mardon skipper. J Insect Conserv 17(1):183–194. https://doi.org/10.1007/s10841-012-9496-x
Jones R, Martinez A, Plackett J, Wainwright D, Ellis S, Kelly C, Hobson R, Wain M, Bourn N, Bulman C (2015) The Changing Status of the High Brown Fritillary Butterfly Argynnis adippe in the UK (1994-2014) Butterfly Conservation Report No. S15-09. Wareham, Dorset
Kittle AM, Fryxell JM, Desy GE, Hamr J (2008) The scale-dependent impact of wolf predation risk on resource selection by three sympatric ungulates. Oecologia 157(1):163–175. https://doi.org/10.1007/s00442-008-1051-9
Kopper BJ, Charlton RE, Margolies DC (2000) Oviposition site selection by the regal fritillary, Speyeria idalia, as affected by proximity of violet host plants. J Insect Behav 13(5):651–665. https://doi.org/10.1023/A:1007887809621
Lele SR, Keim JL, Solymos P (2019) ResourceSelection: Resource Selection (Probability) Functions for Use-Availability Data. R Package version 0.3–5. https://cran.r-project.org/package=ResourceSelection
Maes D, Van Dyck H (2001) Butterfly diversity loss in Flanders (north Belgium): Europe’s worst case scenario? Biol Conserv 99(3):263–276. https://doi.org/10.1016/S0006-3207(00)00182-8
Menéndez R, Gutiérrez D, Thomas CD (2002) Migration and Allee effects in the six-spot burnet moth Zygaena filipendulae. Ecol Entomol 27(3):317–325. https://doi.org/10.1046/j.1365-2311.2002.00404.x
Nakamura Y (2011) Conservation of butterflies in Japan: status, actions and strategy. J Insect Conserv 15:5–22. https://doi.org/10.1007/s10841-010-9299-x
Natural England (2022) National Character Area 20—Morecambe Bay Limestones. https://nationalcharacterareas.co.uk/morecambe-bay-limestones/. Accessed 5 Oct 2022
Nesbitt R (2010) Re-colonisation patterns of the High brown fritillary (Argynis Adippe) following recent habitat restoration. MSc Dissertation. Lancaster University
Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Bocard D, Carvalho G, Chirico M, De Caceres M, Durand S, Beatriz H, Evangilista A, Fitzjohn R, Friendly M, Furneaux B, Hannigan G, Hill MO, Lahti L, Mcglinn D, Ouellette M-H, Ribeiro Cunha E, Smith T, Stier A, Ter Braak CJF, Weedon J (2022) vegan: Community Ecology Package. R Package version 2.6–2. https://cran.r-project.org/package=vegan
Polic D, Tamario C, Franzén M, Betzholtz PE, Yıldırım Y, Forsman A (2021) Movements and occurrence in two closely related fritillary species. Ecol Entomol 46(2):428–439. https://doi.org/10.1111/een.12987
Porter K (1982) Basking behaviour in larvae of the butterfly Euphydryas aurinia. Oikos 38(3):308–312. https://doi.org/10.2307/3544670
R Core Team (2022) R: A Language and Environment for Statistical Computing. Version 4.2.1. https://www.r-project.org/
Rytteri S, Kuussaari M, Saastamoinen M (2021) Microclimatic variability buffers butterfly populations against increased mortality caused by phenological asynchrony between larvae and their host plants. Oikos 130:753–765. https://doi.org/10.1111/oik.07653
Salz A, Fartmann T (2009) Coastal dunes as important strongholds for the survival of the rare Niobe fritillary (Argynnis niobe). J Insect Conserv 13(6):643–654. https://doi.org/10.1007/s10841-009-9214-5
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Singer MC (2004) Oviposition preference: its definition, measurement and correlates, and its use in assessing risk of host shifts. In: Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK (eds) Proceedings of the XI International Symposium on Biological Control of Weeds. CSIRO Entomology, Canberra, pp 235–244
Six A (2000) Zur Populationsbiologie des Großen Perlmuttfalter Argynnis aglaja und des Feurigen Perlmuttfalters Argynnis adippe (Lepidoptera: Nymphalidae). Verhandlungen Des Westdeutschen Entomologentag 1999:81–89
Smee M, Smyth W, Tunmore M, ffrench-Constant R, Hodgson D, (2011) Butterflies on the brink: Habitat requirements for declining populations of the marsh fritillary (Euphydryas aurinia) in SW England. J Insect Conserv 15(1):153–163. https://doi.org/10.1007/s10841-010-9334-y
Stefanescu C, Torre I, Jubany J, Paramo F (2011) Recent trends in butterfly populations from North-East Spain and Andorra in the light of habitat and climate change. J Insect Conserv 15:83–93. https://doi.org/10.1007/s10841-010-9325-z
Thomas JA (1991) Rare species conservation: Case studies of European Butterflies. In: Spellerberg IF, Goldsmith FB, Morris MG (eds) The scientific management of temperate communities for conservation: 31st Symposium: Papers. Blackwell Scientific, Oxford, pp 149–197
Thomas JA, Simcox DJ, Hovestadt T (2011) Evidence based conservation of butterflies. J Insect Conserv 15(1):241–258. https://doi.org/10.1007/s10841-010-9341-z
van Swaay C, Warren M, Loïs G (2006) Biotope use and trends of European butterflies. J Insect Conserv 10(2):189–209. https://doi.org/10.1007/s10841-006-6293-4
van Swaay C, Cuttelod A, Collins S, Maes D, Munguira ML, Sasic M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhoff I (2010) European Red List of Butterflies. Publications Office of the European Union, Luxembourg
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: death by a thousand cuts. Proc Natl Acad Sci U S A 118(2):1–10. https://doi.org/10.1073/PNAS.2023989118
WallisDeVries M (2006) Larval habitat quality and its significance for the conservation of Melitaea cinxia in northwestern Europe. In: Fartmann T, Hermann G (eds) Larvalökologie von Tagfalternund Widderchen in Mitteleuropa. Abhandlungen ausdem Westfalischen Museum fur Naturkunde, pp 281–294
WallisDeVries MF, van Swaay CAM (2006) Global warming and excess nitrogen may induce butterfly decline by microclimatic cooling. Glob Chang Biol 12(9):1620–1626. https://doi.org/10.1111/j.1365-2486.2006.01202.x
Warren MS (1994) Autecology and conservation needs of The High Brown fritillary. Annual Report for 1993/94. English Nature Contract Report No. F72–10–18. Wareham, Dorset
Warren MS (1995a) Managing local microclimates for the high brown fritillary, Argynnis adippe. In: Pullin AS (ed) Ecology and Conservation of Butterflies. Springer, Netherlands, pp 198–210
Warren MS (1995b) Autecology and conservation needs of The High Brown Fritillary. Final Report inc. Annual Report for 1994/95. English Nature Contract Report No. F72–10–18. Wareham, Dorset
Warren MS, Oates MR (1995) The importance of bracken habitats to Fritillary Butterflies and their management and conservation. In: Smith RT, Taylor JA (eds) Bracken: An environmental issue. International Bracken Group, Aberystwyth, pp 178–181
Warren MS, Baker NR, Oates MR (1995) High Brown Fritillary: Site Dossier for Britain 1990–1994. Butterfly Conservation, Wareham, Dorset
Warren MS, Maes D, van Swaay CAM, Goffart P, van Dyck H, Bourn NAD, Wynhoff I, Hoare D, Ellis S (2021) The decline of butterflies in Europe: problems, significance, and possible solutions. Proc Natl Acad Sci U S A 118(2):1–10. https://doi.org/10.1073/PNAS.2002551117
Wepprich T, Adrion JR, Ries L, Wiedmann J, Haddad NM (2019) Butterfly abundance declines over 20 years of systematic monitoring in Ohio, USA. PLoS ONE 14(7):1–21. https://doi.org/10.1371/journal.pone.0216270
Wiklund C (1984) Egg-laying patterns in butterflies in relation to their phenology and the visual apparency and abundance of their host plants. Oecologia 63:23–29. https://doi.org/10.1007/BF00379780
Zimmermann K, Konvička M, Fric Z, Čihaková V (2009) Demography of a common butterfly on humid grasslands: Argynnis aglaja (Lepidoptera: Nymphalidae) Studied by Mark-Recapture. Pol J Ecol 57(4):715–727
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14. https://doi.org/10.1111/j.2041-210x.2009.00001.x
Acknowledgements
Thanks to Martin Wain, David Wainwright, Nigel Bourn (Butterfly Conservation), Chris Winnick (Butterfly Conservation Cumbria Branch) and Simon Spencer for valuable advice and comments. Jane Hopwood, John Garner and National Trust for site access, Natural England for species licencing, and the field assistants who contributed to data collection; Dr Kirsty Godsman, Matthew Bottomley, Dr Nigel Simons, Patrick Taylor, Amy Fawcett, and Gabriel Dixon.
Funding
This study was funded by Edge Hill University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis was performed by JS with input by AO, RM, and PA. The first draft of the manuscript was written by Julia Simons and reviewed and edited by AO, RM, and PA. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
The authors confirm that they follow the rules of good scientific practice and all ethical standards requested by the journal. Ethical approval was received from Edge Hill University research ethics committee, prior to data collection. Collection of habitat data was carried out with licence from appropriate governmental bodies and in an approved and appropriate manner.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simons, J., Oxbrough, A., Menéndez, R. et al. Micro-habitat features determine oviposition site selection in High Brown and Dark Green Fritillaries. J Insect Conserv 27, 841–853 (2023). https://doi.org/10.1007/s10841-023-00503-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00503-w