Abstract
Sampling efficiency, composition and detection biases associated with pitfall-trap and sample plot (standardised hand-collecting) methods were compared at seven high montane sites at Lasha Mountain, Yunnan, China. Rarefaction-interpolation curves showed total species diversity and sample coverage from each method were undifferentiated. Permutational multivariate analysis of variance showed highly overlapping communities. However, of all species collected, just 53% of all species found were shared between the two methods; 30% were exclusive to sample plot samples and 17% were exclusive to pitfall traps. Modelled comparisons of species richness (alpha) and proportion of species from the population (beta diversity) showed differences between methods. Permutational multivariate analysis of variance blocked by site showed community composition differed between methods, defined largely by the presence of a few dominant species.
Implications for insect conservation: Our findings suggest that results from the two methods cannot be directly compared and are imperfect substitutes to one another. For long-term monitoring of biodiversity in complex forest mosaic systems, we suggest integrating a suite of complementary methods to achieve more complete representation of ant composition and diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global biodiversity is declining at an unprecedented rate (IPBES 2019). Monitoring biodiversity is a key part of addressing the trend by providing facts of the occurring changes and mechanisms of change to bring about science-based policy and land management decisions that benefit biodiversity (Noss 1990; Hobbie et al. 2003; Kuussaari et al. 2009; Cardoso et al. 2011; Navarro et al. 2017; Guerra et al. 2021). The continuing challenge is measuring biodiversity in ways that are useful, accurate, and repeatable (Purvis and Hector 2000; Lindenmayer and Likens 2010). Ants, in particular, are an insect group of immense ecological significance, contributing a large proportion of the global arthropod biomass (Schultheiss et al. 2022) and having critical ecological functions such as nutrient cycling, soil aeration, and seed dispersal (de Bruyn and Conacher 1990; Hölldobler and Wilson 1990; Folgarait 1998; Parr et al. 2016; Penn and Crist 2018). Ants are relatively easy to collect, sensitive to environmental change and representative of soil diversity (Andersen et al. 2004; Andersen and Majer 2004; Li et al. 2015a). As such, ants have long been an important insect group for biological monitoring and indicators of ecological change (bioindicators) (Andersen 1997; McGeoch and Chown 1998; Andersen et al. 2002; Underwood and Fisher 2006; Zhang and Ou 2006; Gerlach et al. 2013; Tiede et al. 2017).
Several sampling methods have been developed to collect ants, each with their own strengths and weaknesses (Bestelmeyer et al. 2000). Understanding the limitations to sampling techniques is important to selecting a method, or combinations of methods, that will address survey objectives within a given environment, sampling effort and available expertise (Bestelmeyer et al. 2000; Gotelli et al. 2011; de Souza et al. 2012). To allow stricter comparison of individual studies, numerous researchers have advocated for the adoption of recognised standard protocols (e.g., Li et al. 2009; Antoniazzi et al. 2020). Such protocols have been proposed (e.g., Ants of the Leaf Litter (ALL) Protocol: Agosti and Alonso 2000) and successfully applied (Vineesh et al. 2007; Lopes and Vasconcelos 2008; Bray 2014; Yeo et al. 2017) but universal application has lagged.
In China, the ‘sample-plot’ (样地调查法) is a common method for sampling ants (Xu et al. 1999), followed by pitfall traps. Pitfall traps are small, covered pits set in the ground that target active epigaeic (ground-active) fauna (Bestelmeyer et al. 2000). The widely used method provides relatively simple and cost-effective sampling that allows for continuous day and night sampling (Majer 1997). However, pitfall traps suffer for their sensitivity to species size, activity, and susceptibility (Marsh 1984; Topping and Sunderland 1992; Majer 1997) as well as biases to do with trap diameter, trap depth, spacing and habitat complexity (Luff 1975; Adis 1979; Majer 1997; Ward et al. 2001; Jiménez-Carmona et al. 2020). The sample plot method—a modified ‘direct sampling’ method (Bestelmeyer et al. 2000)—is a technique where specimens are collected systematically from microhabitats using a range of hand-collecting techniques within a specified area and time (Xu 2002). The method has advantages in efficiency but is vulnerable to the competence of the researchers and differences in habitats, reducing the comparability between samples and studies (Bestelmeyer et al. 2000).
Here, we aimed to evaluate these two widely used sample methods in China for long-term ant biodiversity monitoring by assessing their results in terms of ant diversity and community composition in a forest mosaic in Yunling Nature Reserve, Yunnan Province. We expected that sample plot methods would collect more ant species than pitfall traps, where hand sorting could allow for sampling of more cryptic, less abundant and slow-moving ants (Parr and Chown 2001; Longino et al. 2002; de Souza et al. 2012; Mark and Guenard 2017). We also expected that sampling methods may selectively filter the ant community present so that combining methods could improve the survey and generate a closer estimation of true ant community (Longino and Colwell 1997). Our results will provide a measure of the sampling effort required and reliability of the combination of methods to characterise and monitor ground-dwelling ant diversity in a high montane forest mosaic.
Materials and methods
Study area
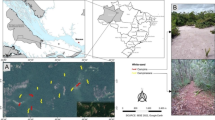
The study was conducted at Lasha Mountain (拉沙山) (N26°20’, E99°15’) in Yunling Nature Reserve, Yunnan, China, within the Mountain Area of Southwest China biodiversity hotspot (Myers et al. 2000). At 75,894 km2, the Yunling Nature Reserve comprises a mosaic of land covers, including subalpine forest, regenerating forest, pastoral grazing land, cropland, and permanent settlements. The study area is a ca. 1400 ha catchment ranging from 2500 to 3700 m asl. Forest vegetation dominates most of the mid-elevations, transitioning from deciduous broadleaved forest (Betula alnoides, Acanthopanax gracilistylus, Acer oliverianum) at lower elevations, through mixed deciduous-conifer forest to conifer forest (Abies georgei, A. fabri, Tsuga dumosa) at the highest elevations. The forest undergrowth primarily comprises Rhododendron spp. and bamboo (Fargesia strigosa, F. edulis, F. solida). Few local families live in the lowest part of the catchment where there is a mixture of grazing, cropland and forest margins. The forest is used by local people for resources such a wood, traditional foods and medicine (Huang et al. 2017). The climate is characterised by alternating dry (winter) and wet (summer) seasons, with an annual mean precipitation of 910 mm (Wang et al. 2012; Li et al. 2019).
Sample design
We sampled ants using two common sampling methods (pitfall trap and sample plot) across the study area. For every 200 m elevation from 2500 to 3700 m, we established one 60 m × 60 m sample site (totalling 7 sites) using the stream as a centreline. Seven pitfall traps were stratified across each site. The open-topped attractant bottles (11 cm in diameter, 15.5 cm in height) were embedded into the ground until flush with the natural soil surface and covered with 15-cm diameter plastic plates to keep rain out. Traps were baited with 50–70 ml liquid attractant composed of 1:1:4:16 solution of ethanol, sugar, vinegar, water (Chen et al. 2011; Li et al. 2017; Fang and Xu 2021). Trap surveys were conducted from August to September in 2018, 2019 and 2020. For each year, traps were shifted to new locations within the sample site for the duration of that year. We emptied traps every two weeks during each sampling period.
Sample plot sampling was undertaken at five 5 m × 5 m sampling plots stratified across each site. After an initial surface inspection, ants were collected by hand from litter, decaying wood and under stones for 24 min (one person) using forceps and aspirators. Using hand tools, soil was excavated to 20 cm to search for and collect ants from subterranean nests. A maximum thirty individuals were collected from a single nest. Foliage collections were made using a 2 m × 2 m white curtain was placed flat around the sample plot and small trees and shrubs up to 5 m were vigorously shaken or beaten to capture ants that fell on the curtain. In total, each 5 m × 5 m plot required approximately 0.5 h to complete, including 24 min of investigation and the time required to return litter, soil or up-turned logs to their original position. Sample plot surveys were completed once for each site in August 2017.
Collected specimens from both methods were stored in 75% ethanol and transferred to a freezer at -10 °C before being identified (Xu 2002).
Statistical analysis
All analyses were performed using R v4.2.0 (R Core Team 2020). Sampling yielded 28 sampling units in total from 21 pitfall trap units and 7 sample plot units. We considered a species x samples matrix with presence-absence data (30 species × 28 samples for all species together).
To visualise the number of species of ants detected with each method, a Venn diagram was drawn using the ‘eulerr’ package in R (Larsson 2021). We drew these for all species, as well as species classed as “dominant”, “common”, and “rare.” We defined dominant species as those with a total relative abundance exceeding 10%; common species contributed between 1% and 10% total abundance, and rare species contributed less than 1% to the total population sampled (Li et al. 2015a).
The effects of method on total measured alpha (α) and beta (β) diversity were tested using a Linear Mixed-Effects Model, using the function lmer in the ‘lme4’ package in R (Bates et al. 2015) with parametric analysis of variance (ANOVA). ‘Site’ was introduced as a random effect in the model. Normality and homogeneity of variance were checked by performing Quartile-Quartile (QQ) plots of the residuals and fitted models using the function plotresid in the package ‘RVAideMemoire’ (Hervé 2018). We identified and removed outliers in the model using the function romr.fnc in the package ‘LMERConvenienceFunctions’ (Tremblay and Ransijn 2015).
We used rarefaction analysis to compare estimated asymptotic species diversity between the sample plot and pitfall trapping methods, a technique based on species frequency (i.e., presence-absence) that avoids biases caused by insufficient or differing sampling efforts (Gotelli and Colwell 2001). To do this, we used sample-sized-based rarefaction-extrapolation analysis based on sampling-unit-based incidence data for each sampling year (Chao et al. 2014a, b). Hill species diversity (or the effective number of species) in the zero (q = 0), first (q = 1), and second (q = 2) orders (or species richness, the exponential Shannon entropy, and inverse Simpson index, respectively) were computed in the ‘iNEXT’ package (Hsieh et al. 2016). In addition, we compared sampling coverage (based on incidence) of each method across Hill species diversities to our sampling effort, permitting estimation of the proportion of the total community represented by the sampling effort and an assessment of sample completeness (sample coverage) across sampling units (Chao and Jost 2012; Chao et al. 2014a; Hsieh et al. 2016). Significant differences in estimated diversity and sampling coverage between methods were judged by non-overlapping confidence intervals (Chao et al. 2014a, b).
To evaluate differences in ant composition of samples obtained from the two methods, we performed non-metric multidimensional scaling (NMDS: calculated with Jaccard dissimilarity index) with the function metaMDS of the R package ‘vegan’ (Oksanen et al. 2019). We considered a species × sample matrix with presence-absence data (30 species × 28 samples). We used presence absence data to avoid assumptions of independence of individual workers (Longino et al. 2002). A dummy species was added to the species matrix to mitigate the impact of pitfall traps that had zero abundances (Clarke et al. 2006). To test for differences between groups (methods), we used a permutational multivariate analysis of variance (PERMANOVA) using distance matrices, which was performed with the function adonis2 in vegan (Oksanen et al. 2019). Because we anticipated assemblages might vary across sites, we conducted the analysis both without and with site as a grouping factor. P values were obtained using 9999 permutations of residuals. To determine which species contributed most to the observed multivariate differences between pitfall trapping and sample plot methods, we used similarity percentage analysis (SIMPER) (Clarke 1993).
Results
Ant collection
Using two sampling methods, we found a total of 10,206 ant (Insecta: Formicidae) specimens belonging to four subfamilies (Myrmicinae, Formicinae, Dolichoderinae, and Ponerinae), 17 genera and 30 species (Table 1; SI 1). Of these, 6312 and 3894 individuals were collected from pitfall trap and sample plot methods respectively. We recorded, 21 species from pitfall traps and 25 species from sample plots, of which only 16 species (53%) were shared (Fig. 1). The most abundant (‘dominant’) species across sampling methods were Pheidole nietneri Emery, 1901, Myrmica rugosa Mayr, 1865, Myrmica kozlovi Ruzsky, 1915, Myrmica bactriana Ruzsky, 1915, Formica fusca Linnaeus, 1758, together accounting for 70% of all the specimens collected (Table 1; Fig. 1). We recorded 11 rare species from pitfall traps and 14 rare species from sample plots, seven of which were shared. Rare species contributed 5% to the total specimen abundance. All species were within their known natural distributions.
Species diversity
Comparisons of observed ant species richness, with ‘site’ as a random factor, showed the sample plot method had higher measured alpha diversity than the pitfall trapping method (Fig. 2; SI 2). The sample plot method also had significantly higher proportion of site-level diversity in samples (beta diversity) in comparison to the pitfall trapping method with the same model (Fig. 2; SI 2). However, rarefaction-extrapolation curves based on inter-annual collections showed ant species diversity across all sites were undifferentiated (over-lapping confidence intervals) for sampling method for all three estimators of diversity (Fig. 3). Total estimated species richness was 35 ± 7.7 s.e. for sample plot method; for pitfall traps, total estimated species richness was 23 ± 7.7 s.e. in 2018, 26 ± 7.3 in 2019, and 24 ± 7.8 s.e. in 2020 (SI 3). Rarefaction results were consistent when comparing diversity at a given sampling effort and coverage (Fig. 3B–C). The sampling coverage completeness for species richness was 76% for the sample plot method, and 82%, 72% and 69% for the pitfall trap method in each year, respectively. To achieve more than 95% sample coverage sample size would need to be increased more than three-times (SI 4).
Sample-based rarefaction (solid lines) and extrapolation (dotted lines) of ants collected using pitfall trap (PF) and sample-plot (SP) methods, with 95% unconditional confidence intervals (shading). Diversity was estimated for species richness, exponential Shannon’s index, and inverse Simpson’s index. (A) A comparison of estimated asymptotic or true diversities for two methods (PF and SP); (B) a comparison of estimated point diversities for sampling units for methods PF and SP; (C) an assessment of sample completeness (sample coverage) across increasing sample units
Community composition
The NMDS plots based on species presence-absence without blocking showed highly overlapping communities (SI 5), whereas NMDS plots showed some clustering according to method when blocked by site (Fig. 4). Accordingly, PERMANOVA results PF and SP assemblages were undifferentiated without blocking, whereas PF and SP assemblages differed significantly when blocked by site (p < 0.05). The NMDS analysis had a low stress value (0.07) which indicates that the ordination summarised the observed distances between samples well (Fig. 4). The SIMPER analysis showed that Myrmica kozlovi, M. bactirana, M. rugosa and Formica fusca contributed the most to the difference in communities between sample methods, collectively contributing 31% to total between method community variation (SI 6). Exclusive species contributed 25% to between method dissimilarity.
Discussion
Our expectation was that sample plot methods would collect more species and accumulation curves would be steeper than those from pitfall traps (Ellison et al. 2007; Abril and Gómez 2013). Our assumption was hand sorting would better target small and slow-moving ants, as well as ants from specific micro-habitats (Andersen 1991; Longino et al. 2002; Véle et al. 2009; Gotelli et al. 2011; de Souza et al. 2012; Mark and Guenard 2017). Instead, comparisons of the efficiency of species accumulation using rarefaction analysis showed interpolated and extrapolated diversity metrics were undifferentiated. Both methods were effective at collecting the most common species across sites, and permutational multivariate analysis across site community assemblages were largely overlapped. However, when we applied ‘site’ as a random factor to our models, differences of alpha and beta diversity and community structure became apparent. At a given site, sample plot methods generally measured a greater species richness, and consequently pitfall traps tended to sample a smaller proportion of the total diversity. Community structure, driven by the relative presences (or absences) of a few dominant and wide-spread forest litter species, differed between methods within sites according to permutational multivariate analysis, suggesting the variation between sites was greater than the variation between methods.
Both methods recovered species undetected by the other method. We found 14 of 30 species were exclusive to one method, representing a range of size-classes, foraging strategies, and nesting behaviours. Contrary to our assumption that pitfall traps would bias large species (Abensperg-Traun and Steven 1995), the proportion of small (2–4 mm) species exclusively collected by each method were comparable, with large (> 5 mm) species represented by Formica sinensis in pitfall collections, and Aphaenogaster lepida and Camponotus anningensis in sample plot collections. In addition, we expected the sample plot method would detect more species that seldom occur above ground, exhibit cryptic feeding behaviour, or occupy small (1–2 m) foraging ranges (Sanders et al. 2007; Véle et al. 2009). Of the exclusive species, only two species exhibiting these traits were observed, one by each method: Ponera bawana, a cryptobiotic foraging species nesting in rotting logs or under stones, was collected in pitfall traps (Taylor 1967; Xu 2001); and Lasius flavus, a hypogaeic (soil-dwelling) species that nests in the soil and under stones and is only rarely observed above ground (Wilson 1955), was detected by hand collection using the sample plot method.
Of exclusive species, we judged 11 to be rare (contributing less than 1% to the total sample). Rare species comprise a high proportion of most highly diverse communities (Novotný and Basset 2000; Burner et al. 2022b) and are important indicators of environmental change (Dopheide et al. 2020). Rare species are also highly sensitive to sampling effort and stochasticity in the environment (Novotný and Basset 2000; Burner et al. 2022a), so that inflating the importance of species appearing in only one sampling method could be misleading. In this study, we estimated sampling effort would need to be doubled to approach 90% sampling coverage of estimated site diversity, and over three times to reach 98% sampling coverage estimated site diversity. With increased sampling or replaying chance encounters, we contend unique (and typically rare) species captured with one sampling method could have also been captured by the other method (Gotelli et al. 2011; Antoniazzi et al. 2020).
A goal of ecological monitoring is comparing year-on-year population or community-level changes (Longino and Colwell 1997; Underwood and Fisher 2006; Lindenmayer and Likens 2010). Comparisons might be to track relative responses to environmental change (e.g., Edwards et al. 2012), or track species interactions (e.g., Sanders et al. 2001; Morgan Ernest et al. 2016). The variety of sampling methods and nuanced protocols add complications when we want to compare results. Long-term monitoring is best accomplished with methods that are, particularly in remote and under-developed regions, simple and cheap, but importantly they need to be robust, repeatable, and comparable. The challenge is that no single method will provide a comprehensive sampling, and each method has its own limitations and biases. For example, Winkler extractions have often been shown to be one of the most effective methods for sampling ants forested ecosystems with substantial litter (e.g., Fisher 1999; Bestelmeyer et al. 2000; Delabie et al. 2000; Martelli et al. 2004; Lessard et al. 2007; Ivanov and Keiper 2009; Ivanov et al. 2009; Sabu et al. 2011); however, subalpine forests, driven by extreme environmental gradients and impacted by centuries of human habitation, often form discontinuous canopies and marginal litter base than more temperate forests. Mature forests are often isolated by non-forested grazing land and intermediate seral communities. Collecting soil monoliths (or turves) offers a robust spatially quantitative method for monitoring soil fauna that is successful across environments (Potapov et al. 2022); samples can be either hand-sorted or extracted using a Berlese-Tullgren funnels (Véle et al. 2009; Yeo et al. 2011; Martins et al. 2020; Basset et al. 2022). Drawbacks are monoliths can be difficult to process in the field, are heavy to transport, and may impact sites long term. Berlese-Tullgren funnels offers a solution to hand-processing (e.g., Barratt et al. 2005; Barratt et al. 2009; Basset et al. 2022) but requires specialised (or purposed-built) equipment, an issue also with using Winkler extractions.
Pitfall traps are cheap, easy to install, and the materials are easily available even in rural China. They are particularly effective in open, less structurally complex vegetation such as grassland, heathland, alpine and savanna ecosystems (Parr and Chown 2001; Steiner et al. 2005; Oliveira et al. 2009; Spotti et al. 2015; Hoffmann and Pettit 2022). It is important to recognise that traps are spatially constrained (Luff 1975; Majer 1997), traps bias active, large-bodied epigaeic species (Abensperg-Traun and Steven 1995), and counts of ants in traps are not independent to one another (Gotelli et al. 2011). Species may vary in their susceptibility to trapping capture, where some species may be more attracted to traps, whereas some species may actively avoid traps (Marsh 1984). The number and composition of ants collected may also be influenced by collection solution (Calixto et al. 2007) or bias resource dominant species where traps are baited (Gotelli et al. 2011). While un-baited traps should improve comparability between studies, we reduced the influence of individual traps by bulking multiple traps.
Active searches, or hand collecting, is an efficient method for recording ant diversity (Andersen 1991; Bestelmeyer et al. 2000; Sanders et al. 2001; Longino et al. 2002; Ellison et al. 2007; Abril and Gómez 2013; Fotso Kuate et al. 2015; Antoniazzi et al. 2020; Massé et al. 2021). Its popularity among scientists is likely stifled because conformity in search time and area protocols are still lacking consensus and concerns for variability in expertise pervade (Gotelli et al. 2011). The sample-plot method is one active species-collection method that employs a standardised set of hand-sampling techniques (i.e., quadrat sampling (Bestelmeyer et al. 2000), nest excavation (Romero and Jaffe 1989), and foliage beating (Harris et al. 1972). It targets available microhabitats—decaying woody material, litter, surface soil and foliage—within a defined space and time (Xu 2002). On a sampling unit basis, we found the combined relative field and laboratory time efficiencies of the sample plot and pitfall trapping methods were comparably similar. One advantage of the sampling plot method we experienced was that field collections were completed in a single site visit, which can be advantageous where sites are remote. Limitations are the sample plot surveys can only be conducted in the daytime (i.e., adequate lighting) and in fair weather (Li et al. 2015b), and hand searches may not be efficient where ants are cryptic or primarily nocturnal. We also found habitats with densely vegetation and abundant coarse woody material were difficult and may (given the time constraint) have been under-sampled in comparison to less complex habitats.
One commonly referred impediment to widespread use of hand collecting is the concern to repeatability between observers. Expert collectors can accumulate species much faster than others and may unconsciously bias novel species by passing over common species (Longino et al. 2002; Sørensen et al. 2009; Gotelli et al. 2011; Antoniazzi et al. 2020). For this reason, Gotelli et al. (2011) suggests naive collectors may be more effective at accumulating species in relative proportion to their true colony densities. Regardless, dealing with the effect of individual collectors can be practically dealt with by with adding “collector” as a random factor to models (Antoniazzi et al. 2020). In our study, sample plot samples were collected using students with direct guidance from a myrmecologist, so we are unable to verify the importance of expertise for completeness or consistency. For remote biological surveys, particularly in areas with prominent local or indigenous groups presence, such as Lasha Mountain, a test for the method will be if long-term biological monitoring can be facilitated by interested locals (Basset et al. 2004). Lasha Mountain has communities representing multiple ethnic nationalities that depend on the local biodiversity and the sustainable management of natural resources for their livelihoods. Establishing methods that can involve indigenous or local people, many of whom have indigenous knowledge, can benefit research as well as improve situations for local people (Braschler et al. 2010).
Another condition for effective long-term biological monitoring is that the methods need to be non-destructive with negligible impact to the local population and supporting habitat (Zaller et al. 2015). This is particularly important in small habitat fragments, sensitive sites, or where species of concern (e.g., ‘threatened’ species) exist (Samways et al. 2010). For example, a common concern of pitfall traps as a monitoring tool is the potential to reduce the overall abundance of invertebrate populations in the local area. Likewise, a strong argument against the use of the sample plot method as a monitoring tool is that repeated soil excavations, destructive searches and collector trampling may have lasting impacts to the micro-habitat, vegetation, and soil. For sample plots, we allowed time to ‘reconstruct’ each plot after intensively searching (turning over logs and rocks, sifting through litter) and excavating soil, although, we cannot be certain the future impacts. We suggest options to modify monitoring frequency or non-destructive alternatives (e.g., Bowie and Frampton 2004) need consideration.
In summary, our results showed both methods are effective for measuring and monitoring ant populations in forest systems. By some metrics, the methods were undifferentiated, but overall selected differently from ant populations. Our conclusion is that the pitfall trap and sample plot methods are effective complements as part of an integrated survey with a low risk of redundancy (Tista and Fiedler 2011; de Souza et al. 2012), giving support to advocates of implementing a diversity of methods, where time labour and expense are not limited (e.g., Bestelmeyer et al. 2000; Basset et al. 2012; de Souza et al. 2012; Antoniazzi et al. 2020; Basset 2020), to improve survey sensitivity and accuracy.
References
Abensperg-Traun M, Steven D (1995) The effects of pitfall trap diameter on ant species richness (Hymenoptera: Formicidae) and species composition of the catch in a semi-arid eucalypt woodland. Aust J Ecol 20:282–287
Abril S, Gómez C (2013) Rapid assessment of ant assemblages in public pine forests of the central Iberian Peninsula. For Ecol Manag 293:79–84
Adis J (1979) Problems of interpreting arthropod sampling with pitfall traps. Zoologischer Anzeiger - A Journal of Comparative Zoology 202:177–184
Agosti D, Alonso LE (2000) The ALL protocol: a standard protocol for the collection of ground-dwelling ants. Ants: standard methods for measuring and monitoring biodiversity, 204–206
Andersen AN (1991) Sampling communities of ground-foraging ants: Pitfall catches compared with quadrat counts in an australian tropical savanna. Aust J Ecol 16:273–279
Andersen AN (1997) Using ants as bioindicators: multiscale issues in ant community ecology. Ecol Soc 1
Andersen AN, Majer JD (2004) Ants show the way down under: invertebrates as bioindicators in land management. Front Ecol Environ 2:291–298
Andersen AN, Hoffmann BD, Müller WJ, Griffiths AD (2002) Using ants as bioindicators in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17
Andersen AN, Fisher A, Hoffmann BD, Read JL, Richards R (2004) Use of terrestrial invertebrates for biodiversity monitoring in australian rangelands, with particular reference to ants. Austral Ecol 29:87–92
Antoniazzi R, Ahuatzin DA, Pelayo-Martínez J, Ortiz-Lozada L, Leponce M, Dáttilo W (2020) On the effectiveness of hand collection to complement baits when studying ant vertical stratification in tropical rainforests. Sociobiology 67:213–222
Barratt BIP, Ferguson CM, Logan RAS, Barton D, Bell NL, Sarathchandra SU, Townsend RJ (2005) Biodiversity of indigenous tussock grassland sites in Otago, Canterbury and the central North Island of New Zealand I. The macro-invertebrate fauna. J Royal Soc New Z 35:287–301
Barratt BIP, Ferguson CM, Barton DM, Johnstone PD (2009) Impact of fire on tussock grassland invertebrate populations. Sci Conserv, pp. 5–75
Basset Y (2020) Suggested field procedures for collecting soil/litter arthropods in a tropical rainforest for long-term monitoring with DNA metabarcoding. protocols.io
Basset Y, Novotny V, Miller SE, Weiblen GD, Missa O, Stewart AJA (2004) Conservation and biological monitoring of tropical forests: the role of parataxonomists. J Appl Ecol 41:163–174
Basset Y, Cizek L, Cuénoud P, Didham RK, Guilhaumon F, Missa O, Novotny V, Ødegaard F, Roslin T, Schmidl J, Tishechkin AK, Winchester NN, Roubik DW, Aberlenc HP, Bail J, Barrios H, Bridle JR, Castaño-Meneses G, Corbara B, Curletti G, Da Rocha WD, De Bakker D, Delabie JHC, Dejean A, Fagan LL, Floren A, Kitching RL, Medianero E, Miller SE, De Oliveira EG, Orivel J, Pollet M, Rapp M, Ribeiro SP, Roisin Y, Schmidt JB, Sørensen L, Leponce M (2012) Arthropod diversity in a tropical forest. Science 338:1481–1484
Basset Y, Hajibabaei M, Wright MTG, Castillo AM, Donoso DA, Segar ST, Souto-Vilarós D, Soliman DY, Roslin T, Smith MA, Lamarre GPA, De León LF, Decaëns T, Palacios-Vargas JG, Castaño-Meneses G, Scheffrahn RH, Rivera M, Perez F, Bobadilla R, Lopez Y, Silva R, Cruz JA, Galván MM, A.A., Barrios H (2022) Comparison of traditional and DNA metabarcoding samples for monitoring tropical soil arthropods (Formicidae, Collembola and Isoptera). Sci Rep 12
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67
Bestelmeyer BT, Agosti D, Alonso LE, Brandao CRF, Brown WL, Delabie JHC, Silvestre R (2000) Field techniques for the study of ground-dwelling ants: an overview, description and evaluation. Ants: standard methods for measuring and monitoring biodiversity, pp. 122–144
Bowie MH, Frampton CM (2004) A practical technique for non-destructive monitoring of soil surface invertebrates for ecological restoration programmes. Ecol Manag Restor 5:34–42
Braschler B, Mahood K, Karenyi N, Gaston KJ, Chown SL (2010) Realizing a synergy between research and education: how participation in ant monitoring helps raise biodiversity awareness in a resource-poor country. J Insect Conserv 14:19–30
Bray D (2014) Modification of the ALL protocol to characterise the overall ant assemblage in temperate eucalypt forest. Australian Entomol 41:15–28
Burner RC, Birkemoe T, Åström J, Sverdrup-Thygeson A (2022a) Flattening the curve: approaching complete sampling for diverse beetle communities. Insect Conserv Divers 15:157–167
Burner RC, Drag L, Stephan JG, Birkemoe T, Wetherbee R, Muller J, Siitonen J, Snäll T, Skarpaas O, Potterf M, Doerfler I, Gossner MM, Schall P, Weisser WW, Sverdrup-Thygeson A (2022b) Functional structure of european forest beetle communities is enhanced by rare species. Biol Conserv 267
Calixto AA, Harris MK, Dean A (2007) Sampling ants with pitfall traps using either propylene glycol or water as a preservative. Southwest Entomol 32:87–91
Cardoso P, Erwin TL, Borges PAV, New TR (2011) The seven impediments in invertebrate conservation and how to overcome them. Biol Conserv 144:2647–2655
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547
Chao A, Chiu C-H, Jost L (2014a) Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu Rev Ecol Evol Syst 45:297–324
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014b) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67
Chen Y-q, Li Q, Wang S-m, Zhou X-y (2011) A comparison of pitfall traps with different liquids for studying ground-dwelling ants (Hymenoptera: Formicidae). Myrmecological News 14:13–19
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80
de Bruyn LAL, Conacher AJ (1990) The role of termites and ants in soil modification: a review. Aust J Soil Res 28:55–93
de Souza JLP, Baccaro FB, Landeiro VL, Franklin E, Magnusson WE (2012) Trade-offs between complementarity and redundancy in the use of different sampling techniques for ground-dwelling ant assemblages. Appl Soil Ecol 56:63–73
Delabie JHC, Fisher BL, Majer JD, Wright IW (2000) Sampling effort and choice of methods. Ants: standard methods for measuring and monitoring biodiversity, 145–154
Dopheide A, Makiola A, Orwin KH, Holdaway RJ, Wood JR, Dickie IA (2020) Rarity is a more reliable indicator of land-use impacts on soil invertebrate communities than other diversity metrics. eLife 9:1–41
IPBES (2019) Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. In: Brondizio ES, Settele J, Díaz S, Ngo HT (eds), p 1148. IPBES secretariat, Bonn, Germany.
Edwards DP, Backhouse AR, Wheeler C, Khen CV, Hamer KC (2012) Impacts of logging and rehabilitation on invertebrate communities in tropical rainforests of northern Borneo. J Insect Conserv 16:591–599
Ellison AM, Record S, Arguello A, Gotelli NJ (2007) Rapid inventory of the ant assemblage in a temperate hardwood forest: species composition and assessment of sampling methods. Environ Entomol 36:766–775
Fang H, Xu ZH (2021) Formicidae insects research of China based on bibliometrics. J Southwest Forestry Univ 41:14
Fisher BL (1999) Improving inventory efficiency: a case study of leaf-litter ant diversity in Madagascar. Ecol Appl 9:714–731
Folgarait PJ (1998) Ant biodiversity and its relationship to ecosystem functioning: a review. Biodivers Conserv 7:1221–1244
Fotso Kuate A, Hanna R, Tindo M, Nanga S, Nagel P (2015) Ant diversity in Dominant Vegetation types of Southern Cameroon. Biotropica 47:94–100
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Gotelli NJ, Ellison AM, Dunn RR, Sanders NJ (2011) Counting ants (Hymenoptera: Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecological News 15:13–19
Guerra CA, Bardgett RD, Caon L, Crowther TW, Delgado-Baquerizo M, Montanarella L, Navarro LM, Orgiazzi A, Singh BK, Tedersoo L, Vargas-Rojas R, Briones MJI, Buscot F, Cameron EK, Cesarz S, Chatzinotas A, Cowan DA, Djukic I, Van Den Hoogen J, Lehmann A, Maestre FT, Marín C, Reitz T, Rillig MC, Smith LC, De Vries FT, Weigelt A, Wall DH, Eisenhauer N (2021) Tracking, targeting, and conserving soil biodiversity: a monitoring and indicator system can inform policy. Science 371:239–241
Harris JWE, Collis DG, Magar KM (1972) Evaluation of the tree-beating method for sampling defoliating forest insects. Can Entomol 104:723–729
Hervé M (2018) RVAideMemoire: testing and plotting procedures for biostatistics. R package version 0.9–69, 3
Hobbie JE, Carpenter SR, Grimm NB, Gosz JR, Seastedt TR (2003) The US Long Term Ecological Research program. Bioscience 53:21–32
Hoffmann BD, Pettit M (2022) Towards precision ecology: Relationships of multiple sampling methods quantifying abundance for comparisons among studies. PLoS One 17
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456
Huang Z-P, Scott MB, Li Y-P, Ren G-P, Xiang Z-F, Cui L-W, Xiao W (2017) Black-and-white snub-nosed monkey (Rhinopithecus bieti) feeding behavior in a degraded forest fragment: clues to a stressed population. Primates
Ivanov K, Keiper J (2009) Effectiveness and biases of winkler litter extraction and pitfall trapping for collecting ground-dwelling ants in northern temperate forests. Environ Entomol 38:1724–1736
Ivanov K, Milligan J, Keiper J (2009) Efficiency of the winkler method for extracting ants (Hymenoptera: Formicidae) from temperate-forest litter. Myrmecological News 13:73–79
Jiménez-Carmona E, Herrera-Rangel J, Renjifo LM, Armbrecht I (2020) Restoration of riparian forest corridors: eight years monitoring the diversity of soil ants in an Andean rural landscape. Insect Conserv Divers 13:384–392
Kuussaari M, Bommarco R, Heikkinen RK, Helm A, Krauss J, Lindborg R, Öckinger E, Pärtel M, Pino J, Rodà F, Stefanescu C, Teder T, Zobel M, Steffan-Dewenter I (2009) Extinction debt: a challenge for biodiversity conservation. Trends in Ecology and Evolution 24:564–571
Larsson J (2021) eulerr: Area-Proportional Euler and Venn Diagrams with Ellipses
Lessard JP, Dunn RR, Parker CR, Sanders NJ (2007) Rarity and diversity in forest ant assemblages of great smoky mountains National Park. Southeast Nat 6:215–228
Li Q, Chen YQ, Xu ZH (2009) Research methods on ant community. Chin J Ecol 28:1862–1870
Li Q, Lu ZX, Zhang W, Ma YY, Feng P (2015a) Communities of ground-dwelling ants in different plantation forests in arid-hot valleys of Jinsha River, Yunnan Province. China Scientia Silvae Sinicae 51:137–145
Li Q, Lu ZX, Zhang W, Ma YY, Feng P (2015b) Ground-dwelling ants as bioindicators during 30-year vegetation restoration in a savanna area, Yunnan. Acta Ecol Sin 35:6199–6207
Li AN, Xu ZH, Xu GL, Zheng Y, Zhuang JX, Li B (2017) Ant diversity of Tongbiguan Nature Reserve and adjacent area in Yunnan. J Southwest Forestry Univ 37:135–141
Li N, Huang Z-P, Xiao W, Cui L-W (2019) Seed dispersal by black-and-white snub-nosed monkey (Rhinopithecus bieti) at Lashashan, Yunnan, China. Chin J Ecol 8:2377–2381
Lindenmayer DB, Likens GE (2010) The science and application of ecological monitoring. Biol Conserv 143:1317–1328
Longino JT, Colwell RK (1997) Biodiversity assessment using structured inventory: capturing the ant fauna of a tropical rain forest. Ecol Appl 7:1263–1277
Longino JT, Coddington J, Colwell RK (2002) The ant fauna of a tropical rain forest: estimating species richness three different ways. Ecology 83:689–702
Lopes CT, Vasconcelos HL (2008) Evaluation of three methods for sampling ground-dwelling ants in the brazilian Cerrado. Neotrop Entomol 37:399–405
Luff ML (1975) Some features influencing the efficiency of pitfall traps. Oecologia 19:345–357
Majer J (1997) The use of pitfall traps for sampling ants - a critique. Mem Museum Vic 56:323–329
Mark KL, Guenard B (2017) Subterranean ants: summary and perspectives on field sampling methods, with notes on diversity and ecology (Hymenoptera: Formicidae). Myrmecological News 25:1–16
Marsh A (1984) The efficacy of pitfall traps for determining the structure of a desert ant community. J Entomol Soc South Afr 47:115–120
Martelli MG, Ward MM, Fraser AM (2004) Ant diversity sampling on the southern Cumberland Plateau: a comparison of litter sifting and pitfall trapping. Southeast Nat 3:113–126
Martins MFO, Thomazini MJ, Baretta D, Brown GG, da Rosa MG, Zagatto MRG, Santos A, Nadolny HS, Cardoso GBX, Niva CC, Bartz MLC, Feitosa RM (2020) Accessing the subterranean ant fauna (Hymenoptera: Formicidae) in native and modified subtropical landscapes in the neotropics. Biota Neotrop 20
Massé PSM, Tadu Z, Djieto Lordon C, Mony R, Kenne M, Tindo M (2021) Efficiency of sampling methods for capturing soil-dwelling ants in three landscapes in southern Cameroon. SOIL ORGANISMS 93:115–132
McGeoch MA, Chown SL (1998) Scaling up the value of bioindicators. Trends in Ecology and Evolution 13:46–47
Morgan Ernest SK, Yenni GM, Allington G, Christensen EM, Geluso K, Goheen JR, Schutzenhofer MR, Supp SR, Thibault KM, Brown JH, Valone TJ (2016) Long-term monitoring and experimental manipulation of a Chihuahuan desert ecosystem near Portal, Arizona (1977–2013). Ecology 97:1082
Myers N, Mittermeler RA, Mittermeler CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Navarro LM, Fernández N, Guerra C, Guralnick R, Kissling WD, Londoño MC, Muller-Karger F, Turak E, Balvanera P, Costello MJ, Delavaud A, Serafy E, Ferrier GY, Geijzendorffer S, Geller I, Jetz GN, Kim W, Kim ES, Martin H, McGeoch CS, Mwampamba MA, Nel TH, Nicholson JL, Pettorelli E, Schaepman N, Skidmore ME, Pinto ASousa, Vergara I, Vihervaara S, Xu P, Yahara H, Gill T, M., Pereira HM (2017) Monitoring biodiversity change through effective global coordination. Curr Opin Environ Sustain 29:158–169
Noss RF (1990) Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv Biol 4:355–364
Novotný V, Basset Y (2000) Rare species in communities of tropical insect herbivores: pondering the mystery of singletons. Oikos 89:564–572
Oksanen J, Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin P, O’Hara R, Simpson G, Solymos P (2019) vegan: Community Ecology Package. R package version 2.5-6
Oliveira M, Lucia TMC, Marinho C, Delabie J, Morato ER (2009) Ant diversity in an area of the Amazon Forest in acre, Brazil. Sociobiology 54:243–267
Parr CT, Chown SL (2001) Inventory and bioindicator sampling: testing pitfall and winkler methods with ants in a south african savanna. J Insect Conserv 5:27–36
Parr C, Eggleton P, Davies A, Evans TA, Holdsworth S (2016) Suppression of savanna ants alters invertebrate composition and influences key ecosystem processes. Ecology 97:1611–1617
Penn HJ, Crist TO (2018) From dispersal to predation: a global synthesis of ant–seed interactions. Ecol Evol 8:9122–9138
Potapov AM, Sun X, Briones MJI, Brown GG, Cameron EK, Cortet J, Eisenhauer N, Fujii S, Geisen S, Gongalsky KB, Guerra C, Handa IT, Janion-Scheepers C, Lindo Z, Mathieu J, Murvanidze M, Nielsen UN, Scheu S, Schmidt O, Schneider C, Seeber J, Tuma J, Tiunov AV, Zaitsev AS, Wall DH (2022) Global monitoring of soil animal communities using a common methodology
Purvis A, Hector A (2000) Getting the measure of biodiversity. Nature 405:212–219
R Core Team (2020) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Romero H, Jaffe K (1989) A comparison of methods for sampling ants (Hymenoptera, Formicidae) in savannas. Biotropica 21:348–352
Sabu TK, Shiju RT, Vinod K, Nithya S (2011) A comparison of the pitfall trap, Winkler extractor and Berlese funnel for sampling ground-dwelling arthropods in tropical montane cloud forests. J Insect Sci 11
Samways MJ, McGeoch MA, New TR (2010) Insect conservation: a handbook of approaches and methods. Oxford University Press
Sanders NJ, Barton KE, Gordon DM (2001) Long-term dynamics of the distribution of the invasive Argentine ant, Linepithema humile, and native ant taxa in northern California. Oecologia 127:123–130
Sanders NJ, Gotelli NJ, Wittman SE, Ratchford JS, Ellison AM, Jules ES (2007) Assembly rules of ground-foraging ant assemblages are contingent on disturbance, habitat and spatial scale. J Biogeogr 34:1632–1641
Schultheiss P, Nooten S, Wang R, Wong M, Brassard F, Guénard B (2022) The abundance, biomass, and distribution of ants on Earth. Proc Natl Acad Sci 119, e2201550119
Sørensen LI, Mikola J, Kytöviita MM, Olofsson J (2009) Trampling and spatial heterogeneity explain decomposer abundances in a sub-arctic grassland subjected to simulated reindeer grazing. Ecosystems 12:830–842
Spotti FA, Castracani C, Grasso DA, Mori A (2015) Daily activity patterns and food preferences in an alpine ant community. Ethol Ecol Evol 27:306–324
Steiner FM, Schlick-Steiner BC, Moder K, Bruckner A, Christian E (2005) Congruence of data from different trapping periods of ant pitfall catches (Hymenoptera: Formicidae). v. 46
Taylor RW (1967) A monographic revision of the ant genus Ponera Latreille (Hymenoptera: Formicidae). Pac Insects Monogr 13:1–112
Tiede Y, Schlautmann J, Donoso DA, Wallis CI, Bendix J, Brandl R, Farwig N (2017) Ants as indicators of environmental change and ecosystem processes. Ecol Ind 83:527–537
Tista M, Fiedler K (2011) How to evaluate and reduce sampling effort for ants. J Insect Conserv 15:547–559
Topping CJ, Sunderland KD (1992) Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. J Appl Ecol 29:485–491
Tremblay A, Ransijn J (2015) LMERConvenienceFunctions: Model selection and post-hoc analysis for (G) LMER models. R package version, 2
Underwood EC, Fisher BL (2006) The role of ants in conservation monitoring: if, when, and how. Biol Conserv 132:166–182
Véle A, Holuša J, Frouz J (2009) Sampling for ants in different-aged spruce forests: a comparison of methods. Eur J Soil Biol 45:301–305
Vineesh PJ, Sabu TK, Karmaly KA (2007) Community structure and functional group classification of litter ants in the montane evergreen and deciduous forests of Wayanad region of western ghats, Southern India. Orient Insects 41:427–442
Wang SJ, Huang ZP, He YC, He XD, Li DH, Sun J, Cui LW, Xiao W (2012) Mating behavior and birth seasonality of black-and-white snub-nosed monkeys (Rhinopithecus bieti) at Mt. Lasha. Zoological Res 33:241–248
Ward DF, New TR, Yen AL (2001) Effects of pitfall trap spacing on the abundance, richness and composition of invertebrate catches. J Insect Conserv 5:47–53
Wilson EO (1955) A monographic revision of the ant genus Lasius. Bull museum Comp Zool Harv 113:2pls
Xu Z (2001) Four new species of the ant genus Ponera Latreille (Hymenoptera: Formicidae) from Yunnan, China. Entomotaxonomia 23:217–226
Xu Z (2002) A study on the biodiversity of Formicidae ants of Xishuangbanna Nature Reserve. Yunnan Science and Technology Press, Kunming, pp 15–67
Xu Z-h, Zeng G, Liu T-y, He Y-f (1999) A study on Communities of Formicidae Ants in different subtypes of Vegetation in Xishuangbanna District of China. Zoological Res 20:118–125
Yeo K, Konate S, Tiho S, Camara SK (2011) Impacts of land use types on ant communities in a tropical forest margin (Oumé - Côte d’ivoire). Afr J Agric Res 6:260–274
Yeo K, Delsinne T, Konate S, Alonso LL, Aïdara D, Peeters C (2017) Diversity and distribution of ant assemblages above and below ground in a west african forest–savannah mosaic (Lamto, Côte d’Ivoire). Insectes Sociaux 64:155–168
Zaller JG, Kerschbaumer G, Rizzoli R, Tiefenbacher A, Gruber E, Schedl H (2015) Monitoring arthropods in protected grasslands: comparing pitfall trapping, quadrat sampling and video monitoring. Web Ecol 15:15–23
Zhang HY, Ou XH (2006) Using insect for indicator to monitor and assess forest ecosystem health. World Forestry Research 19:22–25
Acknowledgements
This study was supported by Ten Thousand Talents Plan of Yunnan (YNWR-CYJS-2018-052) and Yunnan Intelligence Union Program (202203AM140015). The authors would like to express their gratitude to the Institute of Eastern-Himalaya Biodiversity Research and the Administration of Yunling Provincial Nature Reserve. We gratefully acknowledge the invaluable help of Professor. Zheng-Hui Xu, Southwest Forestry College for species identification and assistance in the field.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Z.C.J. and C.Y.T. completed field work. M.B.S., Z.C.J., and C.Y.T. wrote the main manuscript text. M.B.S. completed analysis and prepared figures. All authors contributed original concepts and ideas. All authors reviewed manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhang Chuan-Jing and Cheng Yi-Ting Contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, CJ., Cheng, YT., Luo, XS. et al. Quantifying ant diversity and community in a subalpine forest mosaic: a comparison of two methods. J Insect Conserv 27, 813–824 (2023). https://doi.org/10.1007/s10841-023-00501-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00501-y