Abstract
The wild bee community of a sand steppe habitat in Eastern Austria was surveyed in the years 2018 and 2019, complemented with historical data from over 100 years, and analyzed in relation to land use change. The mapping of land use categories was based on historical aerial photographs and orthophotos. Changes in bee community composition were analyzed by a multivariate statistical approach and took ecological traits into account (lecty, nesting type, habitat requirements, flight period, parasitism). In total, 310 bee species were recorded in the area, with the oldest records dating back to 1882. The bee species composition differed significantly among four defined time periods. Across the two most intensively sampled time periods (1931–1966 vs 2001–2021), a decline in species richness of over 50% was observed. We observed a disproportionally high decline of steppe- and sand-associated species, and a distinct shift from ground nesting species to above-ground nesting species. The area covered with woods increased from 1966 to 2018, while the total area covered with grassland and fallows decreased slightly between 1966 and 1994. The oligolectic species assemblage was specialized on Dipsacaceae, Brassicaceae and Fabaceae in the two earlier periods, and on Asteraceae and specifically on Carduoideae during the two later ones.
Implications for insect conservation
stronger reference to historic land management practices as short time periods of intensive grazing and small-scaled, staggered mowing would be desirable to improve the habitat quality. More drastic measures, such as removal of the topsoil in some parts and changes in the landscape re-establishing exposure to wind erosion, might be necessary, if the area is to be fully restored to the condition it was in a century ago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild bees are among the most important pollinators of insect-pollinated wild and cultivated plants (Klein et al. 2007; Ollerton et al. 2011). Protection and successful conservation of wild bees are of paramount importance to ensure human well-being (Vanbergen et al. 2013) and biodiversity of organisms in other trophic levels (Potts et al. 2010). The close and often highly specialized relationships between bees and plants also imply a risk of cascading extinctions with severe consequences for the resilience of ecosystem functions (Potts et al. 2010; Vanbergen et al. 2013; IPBES 2016). Habitat destruction, fragmentation, and degradation along with land use change and intensive land management practices have been identified as main drivers of pollinator decline on a global scale (Goulson et al. 2015; IPBES 2016; Sánchez-Bayo and Wyckhuys 2019). However, variation in the trends of biodiversity loss highlights the need to address these complex relationships with detailed studies of realm-, region- and taxa-specific trends over time (Dornelas and Daskalova 2020).
In Europe, natural and semi-natural grasslands, traditionally used for livestock grazing, have been the subject of a dramatic decrease (Dengler et al. 2014), with an area reduction of more than 80% between 1960 (289,809 ha) and 2020 (53,171 ha) in Austria (Grüner Bericht 2022). Particularly natural steppes and semi-dry grasslands are highly endangered through transformation into arable fields, afforestation, abandonment, eutrophication or biotic invasion (Vrahnakis et al. 2013). In Central Europe these habitats, on which many endangered xerothermic bee species critically depend, have been reduced to small, protected sites (Wiesbauer 2008, 2020; WallisDeVries and van Swaay 2009; Dengler et al. 2014; Nieto et al. 2014).
The sandy steppes in the eastern Marchfeld Plain are the last remnants of a once extensive dune landscape formed during the last ice age and at the beginning of the postglacial period. During this period the water level dropped, exposing previously submerged banks and allowing the wind to spread large quantities of fine sediment in the landscape (Küster 1999; Wiesbauer and Mazzucco 1997). Today, non-forested sand areas are only found very locally in Lower Austria. “Pannonic sand steppes” are among the most endangered habitats in Austria and home to a unique fauna and flora. For several plant and animal species specialized on sandy soil, the study site “Sandberge Oberweiden” is or has until recently been the last refuge in Austria (Kasy 1957; Wiesbauer and Mazzucco 1997; Rabitsch 2002). Thus, the “Pannonic sand steppes” are priority habitats according to the Fauna-Flora-Habitat Directive and therefore under special protection (European Council 2007).
In the present study we survey the nature reserve “Sandberge Oberweiden”, a 126-hectare large sand steppe habitat in Eastern Austria, which is protected since 1961 (Wiesbauer 2002a). The unique steppe fauna and flora of Oberweiden has been known since the end of the nineteenth century (Kasy 1957). The oldest bee records from the area are specimens collected by the Austrian entomologists Josef Kolazy and Anton Handlirsch in 1882, followed by records from Hans Zerny around 1915 (specimens in Coll. NHMW), while the most comprehensive historical data go back to the Austrian bee researchers Bruno Pittioni and Stefan Schmidt in the 1930s and 1940s (Pittioni and Schmidt 1942, 1943; Funnell 2022). In the 1950s and 1960s the site was occasionally studied by hymenopterologists from Upper Austria, foremost Andreas W. Ebmer and Josef Gusenleitner (Zobodat 2021).
The long history of entomological research on the site provides a rare opportunity to understand changes in the species composition over a longer period of time. Among the existing studies, there is only a small number addressing such changes; e.g. in the biodiversity database BioTIME only 1.4% of the time series reach back over 50 years (Dornelas et al. 2018). Based on museum records, Bartomeus et al. (2013) analyzed historical changes over 140 years in northeastern US bee fauna related to shared ecological traits and found correlations of the dietary and phenological breadth with species abundance. In a global analysis based on records from the Global Biodiversity Information Facility, Zattara and Aizen (2021) found a distinct decline in species richness since the 1990s and demonstrated patterns of species richness correlated with the phylogenetic structure of bees. There are several studies correlating bumblebee species richness and abundance with different management regimes in grasslands and the agricultural landscape (e.g. Carvell 2002; Carvell et al. 2004; Bommarco et al. 2012). However, bumble bees have been shown to react differently to changes in landscape structure than other wild bee guilds (Steffan-Dewenter et al. 2002), so that the conclusions of these studies are to be considered separately. Finally, there are also a few studies focusing on effects of changing anthropogenic disturbance (Winfree et al. 2009; Williams et al. 2010) or agricultural practices and structures (Kennedy et al. 2013) over longer time periods.
In the present study we combine several methods to display and explain the loss of biodiversity in a geographically restricted area of high conservation value. Modern records, mainly consisting of a recent survey of wild bees, and older records retrieved from collections, literature and databases are pooled in four time periods (before 1930, 1931–1966, 1967–2000, 2001–2021) and compared in terms of species richness and community composition focusing on ecological traits of the species. The results and changes are analyzed in relation to changes in land use, extracted from historical aerial photos and recent orthophotos. Specifically, we asked (i) whether the bee community changed in relation to ecological traits over time (lecty, nesting type, habitat requirements, flight period, parasitism), (ii) how land use differed between the four time periods and (iii) if changes in the wild bee community were correlated to land use change and can be explained by specific drivers.
Materials and methods
Study area
The study area (Fig. 1A; N 48° 17′ 13′′, E 16° 49′ 43′′) is located in Lower Austria, south of the village Oberweiden. It is part of the Natura 2000 protected area “Pannonische Sanddünen” (2504.97 ha) and the protected habitat type “Pannonic sand steppes” (European Environment Agency 2022).
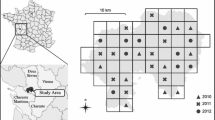
The study site “Sandberge Oberweiden” A location of the study site; protected areas are marked in green, the transect of the landscape study is marked by a red square, and the village Oberweiden by a star symbol, B overview of study plots, C sandy area on the former racing course, D bare ground patches on the “Hills”. HG Hills, RB former horse racing course, AF old area, NF new area (data source hillshade and orthophoto: basemap.at, nature conservation areas: Abteilung RU5—Naturschutz/Amt der NÖ Landesregierung—https://www.noe.gv.at/, municipal boundaries: Abteilung BD1—GIS Support/Amt der NÖ Landesregierung—https://www.noe.gv.at/. (Colour figure online)
For the apidological survey (2018–2019), the 126-hectare large area was divided into four study plots (Fig. 1B) representing differently structured parts of the landscape: the Hills in the northern part of the reserve (HG), the Old Area west of the Hills (AF), the New Area which has only recently been set under protection (NF) and is adjacent to the Old Area to the northwest, and a former horse racing course, which occupies a large area to the south (RB). Important foraging plants were qualitatively (i.e. not systematically) surveyed in the area to improve the definition of the four study plots.
Hills (Fig. 1B: HG, 1D)
The hilly area in the northern part of the site provides a suitable habitat for many rare plants, such as the common gypsophila Gypsophila paniculata, the late carnation Dianthus serotinus, and the immortelle Helichrysum arenarium. Other flowering plants of interest to wild bees include knapweeds Centaurea sp., field eryngo Eryngium campestris, and Odontites luteus. In the southern part, where the Hills gradually transition into the flat, steppe-like meadow, the ground is sparsely vegetated. The open, sandy areas serve as nesting habitat for various species of wild bees and wasps. Prickly saltwort Kali turgidum grows on the open, sparsely vegetated soil patches. The density of flowers differs between seasons, and occasionally the grasses grow tall. Partly, the hills have become overgrown by bushes as they are obviously not included in the mowing cycle, as the slope makes machine mowing difficult (pers. obs.).
Former horse racing course (Fig. 1B: RB, 1C)
This study plot is an extensive steppe-like area surrounded by the track of a former horse racing course. The race course itself is covered with loose sandy soils. Its edges form a low, sandy slope towards the meadow of the “Hills” area and are partially covered with flowering plants such as thyme Thymus sp., hoary alyssum Berteroa incana, yellow scabious Scabiosa ochroleuca, and knapweed Centaurea sp.. Hoary alyssum seems to be particularly attractive to various wild bee species, flowering in high abundance, especially in the southeast corner of the horse track. Inside the racing circle, different grasses constitute the dominant vegetation. Very early in the season, sand cinquefoil Potentilla incana flowers in large numbers.
Old area (Fig. 1B: AF)
The Old Area is a meadow west of the Hills, crossed by several sandy paths, which provides suitable nesting sites for solitary bees. Flowering plant species include knapweed Centaurea sp., thorny knapweed Ononis spinosa, and resede Reseda sp., but the dominant vegetation are again grasses.
New area (Fig. 1B: NF)
North of the Old area and the Hills is another meadow that has only recently become a part of the nature reserve. The area harbours a diversity of flowering plants, such as thorny knapweed Ononis spinosa, yellow scabiosa Scabiosa ochroleuca, knapweed Centaurea sp. and sage Salvia sp. This area is more densely covered with vegetation and is almost devoid of bare ground patches for ground-nesting bees.
Today, the protected area is managed by mowing. As a result of an EU LIFE-project (Wiesbauer 2002b) alternating staggered mowing with a certain proportion of the area left uncut each year was proposed. Nevertheless, own and third-party observations (e.g. M. Lödl pers. comm.) have shown that nearly the entire area is actually mowed in one go by selecting the overlapping time window for mowing that is open for all sub-areas in accordance with the management contract. Moreover, instead of rotating the uncut areas annually to a different patch and thus preventing scrub encroachment, simply the steep slopes are left uncut every year, which were at the time of the study covered by dense vegetation and scrubs. The management plan is currently being revised (pers. comm. Department of Nature Conservation, State Government of Lower Austria).
Field work
Surveys were conducted by hand-netting from April until September 2018 and from April until August 2019 (for dates see Online Resource 1). All specimens are deposited in the Natural History Museum Vienna. For the determination of the species, Amiet et al. (1999, 2001), Dathe et al. (2016), Ebmer (1969, 1970, 1971, 1973), Scheuchl (1995, 1996), Schmid-Egger and Scheuchl (1997), as well as the Collections of the Natural History Museum Vienna (NHMW) and the authors (SS, HZ) were used. A complete list of the collected specimens including specimen-IDs and GPS coordinates is given as supplementary material (Online Resource 1).
Bumblebees (Bombus spp.) were not considered in the most recent survey (2018–2019). However, historical bumblebee records are included in Online Resource 2, but not further evaluated or discussed in comparison with recent data.
Nomenclature
Nomenclature follows Scheuchl and Willner (2016), with the following exceptions: classification of Eucera s.l. follows Dorchin et al. (2018), Andrena afzeliella is understood following Praz et al. (2022). If a species is mentioned under an older name in literature, it is indicated in the respective reference in Online Resource 2, while the record is listed under the currently valid name.
Databases, literature and collection data
The data from the current survey were complemented by historical data from the Collection of the Natural History Museum Vienna (NHMW Coll.), the Collection of the Landesmuseum Burgenland (Coll. Bgl.), the database of the Natural History Museum Vienna (NHMW-DB), the Global Biodiversity Information Facility (GBIF 2022), the Zobodat specimen database (Zobodat 2021), and the private database of Herbert Zettel (HZ-DB in Online Resource 2), as well as for selected species the bee database Apidat owned by the Biologiezentrum Linz, and finally the Pittioni Bee Collection Index Cards held at and digitized by the Natural History Museum, London (Pittioni Index Cards, Funnell 2022) and a comprehensive literature search using the Zobodat literature database (Zobodat 2021). All literature references used for compiling the species list in Online Resource 2 are listed there.
Bruno Pittioni and Stefan Schmidt (1942, 1943) published an extensive survey of the bee fauna of Eastern Austria. For many species they listed all known records, but for more common species the localities were listed without reference to specific records. In cases in which such records were found in the Pittioni Index Cards, these were prioritized. The high number of recorded species in the year 1942 is due to the records published by Bruno Pittioni (1942) without a year date as these records were assigned the year of publication. However, they may have been collected earlier.
Comparability of historical and recent data
Neither the historical data, nor the data acquired during the recent survey originate from a standardized survey and are thus subject to potential biases. Only the survey conducted by Bruno Pittioni and Stefan Schmidt in the 1930s and 1940s (time period 1931–1966) and the most recent time period (2001–2021) can be assumed to provide an approximately complete list of species. In between (1967–2000), the site was only surveyed occasionally by bee researchers from Linz (Upper Austria).
Another limitation of the study is that the determinations of the historical material were not checked. However, the majority of the data sources from Pittioni and Schmidt, who were very experienced bee researchers, is assumed to be identified correctly. Likewise, S. Schoder, who did the majority of the field work and determination, is very experienced in collecting and determining bees, so that the current dataset can be assumed to be rather complete and reliable and thus well comparable with the historical data. The same is true for H. Zettel, whose data were added, and most of the data present for the time period 1967–2000. No differences are to be assumed in the method of collecting specimens with a hand net. Questionable specimen records, e.g. due to an unlikely habitat association and a lack of a voucher specimen, were excluded from the species list.
The notably low number of species in the genus Hylaeus in the historical data could be due to the fact that there was no key for the genus at that time. In addition, several species were later re-splitted (Dathe 1980; Dathe et al. 2016; Straka & Bogusch 2011). Additionally, the expertise of one of the authors of the present study (SS) on Hylaeus could contribute to a more complete species list. Likewise, taxonomical changes occurred in the genus Colletes concerning the C. marginatus and C. chengtehensis species complex. However, historical and recent material was evaluated in a recent publication (Zettel et al. 2019a), showing that in the study region only one species occurs (C. chengtehensis).
Finally, due to imprecise locality on the historical specimen labels, it must be assumed that until the 1960s, records labelled as “Oberweiden” do not solely refer to the protected area as defined today, but also potentially include the area between the steppe habitat and the train station in the village a few kilometres away, where entomologists arrived (Fig. 1A). For this reason, the entire area was considered in the landscape analysis.
Assignment of ecological traits
The ecological traits, specifically feeding preferences (i.e. lecty: polylectic, oligolectic), preferred plant taxa of oligolectic species, nesting type (ground nesting vs above-ground nesting species; above-ground includes nesting in dead wood, plant stems, beetle tunnels, cavities, snail shells and freely suspended), parasitism (nest-building vs parasitic species), host associations for parasitic species, habitat requirement (open land, forest edge, ubiquitous) and strong dependence on sand or steppe-like habitats were assigned following Scheuchl and Willner (2016) and Wiesbauer (2020). Additionally, the species were phenologically grouped according to their main flight period: Univoltine species active from March onwards were defined as spring species, while species recorded between May and September were grouped as summer species and those active from August onwards as autumn species. Further, species flying from April or earlier until August or later were classified as species with a long flight period, which include bivoltine species or eusocial species. The flight time data from Scheuchl and Willner (2016) were used as the basis for this assessment. Since these data seem to include also very early and late appearances of the species, the flight times in the respective groupings appear quite long. Overall, however, the assignments of the species to the groups also correspond to our experience.
Landscape study
To correlate changes in wild bee community composition with temporal land use change we mapped land use categories (Table 1) from historical aerial photos and recent orthophotos. Historical aerial photos were georeferenced before mapping. Georeferencing and landscape mapping was done with ArcGIS Pro 2.8 (ESRI 2021). Historical aerial photos/orthophotos were retrieved from the Austrian Federal Office of Metrology and Surveying (BEV, Bundesamt für Eich- und Vermessungswesen) and were available for 1966, 1994 and 2000. The most recent orthophoto, when conducting the study, was from 2018 (basemap.at s.a.). The land use categories were defined based on habitat requirements of wild bees, preliminary mapping of the 1966 aerial photo and expert discussion within the author team. Because of the above-mentioned uncertainty of location of specimen records until the 1960s, the geographical frame for landscape mapping was extended from the protected area northwards to include the train station of Oberweiden (Fig. 1A).
Data analysis
All data analyses were done using the software R version 4.1.2 (R Core Team 2021). All wild bee data were analyzed as presence-absence data. The proportion of coverage per landscape category per year (1966, 1994, 2000, 2018) was calculated in ArcGIS Pro 2.8. Changes of wild bee species richness per trait and land use changes between the mapped years were visualized with a stacked area chart with the R-package ggplot2 (Wickham 2016). To provide a measure for species turnover across all time periods, the Sørensen index was calculated in the R package vegan (Oksanen 2013; Oksanen et al. 2020).
The wild bee data from periods with approximately complete sampling (1931–1966 and 2000–2021) are compared descriptively. Due to very sparse wild bee data availability in some observation years, specifically between 1885 and 1930 as well as between the 1950s and the 2010s, these data were pooled (Table 2) for statistical analysis. Consecutively sampled years with species richness < 10 were pooled to result in groups of at least 10 species. Periods separated by apparent sampling gaps were not pooled (e.g. the 10 years gap between 1888 and 1908). Further, longer periods or decades (e.g., 1908–1930, 1950–1959, 1960–1969) mostly consisting of several consecutive sampling years with low species richness (< 7 spp.) interspersed with single sampling years comprising high species richness (e.g., 1911 with 33 spp.; 1952 with 16 spp.; 1967 with 23 spp.) were pooled. Additionally, the data’s historical background was considered by handling the 1930s and 1940s data, collected by Bruno Pittioni, as separate as possible (Pittioni and Schmidt 1942, 1943). From the 1950s onwards, the availability and relation of land use was also considered in the pooling process. For analyzing the subsets of wild bee species specialized on sand and steppe habitats and oligolectic species the pooling was slightly adapted to accomplish with the previous explained data pooling approach (e.g., years had to be dropped due to the reduction of species with specialization).
To show differences of the wild bee community over time and relations to land use change, the data were attributed to time periods (before 1930, 1931–1966, 1967–2000, 2001–2021) in accordance with the available historical aerial photos/orthophotos (Table 2). Since the relation of wild bee data before 1950 with a landscape situation from 1966 (earliest aerial photo available for the region) seemed inadequate, no landscape data were attributed to wild bee data before 1950 (Table 2).
To analyze changes in wild bee community composition, traits and related land use change, a multivariate statistical approach was chosen. We performed a NMDS (non-metric multidimensional scaling) with the R package vegan (Oksanen et al. 2020) using a binary bray–curtis distance matrix to show differences in the wild bee community among the defined time periods (Table 2). To analyze how ecological traits were associated over time, community weighted means (CWMs) per observation (year or aggregated observation years; Table 2) were calculated with the R package FD (Laliberté et al. 2014; Laliberté and Legendre 2010) using the ecological traits: nesting type, lecty, parasitism, flight period and habitat requirements. The CWMs and the proportions per land use category were fitted onto the NMDS using the function “envifit” of the R package vegan (Oksanen et al. 2020). Some traits appeared to be dominant over time (nest building vs parasitism, nesting type; Table 2), thus these CWMs had to be excluded from this fitting step. Due to significant heterogeneity of the wild bee community among the time periods (permutation test in vegan: 999 permutations, p = 0.001) we did not perform an ADONIS to assess significant variation among the time periods, but instead we included the time periods in the vector fitting.
Results
Distribution and composition of species records
Altogether 310 wild bee species representing 41 genera have been recorded in and around the nature reserve Oberweiden (Online Resource 2). The oldest records date back to the year 1882. When it comes to the collecting efforts, the data are distributed unequally, with more than 15 species recorded for the years 1885 (17 spp.), 1911 (33 spp.), 1934–1938 (45, 59, 34, 48 and 38 spp.), 1942–1943 (115 and 37 spp.), 1952 (16 spp.), 1959 (35 spp.), 1967 (23 spp.) and 2018–2020 (52, 87 and 28 spp.). 99 species were recorded during the recent survey (2018–2019) by DZ and SS. Across the entire time period of the dataset a high species turnover (Sørensen index = 0.88) was detected.
Across the four time periods and excluding the 21 documented bumblebee species (Bombus spp.), 79 of 289 species were recorded before 1930, 246 in the period 1931–1966, 34 between 1967 and 2000, and 119 between 2001 and 2021. 160 of the 289 species have not been recorded after 1966 and 140 (48.4%) not after 1952. Eight species were recorded in all four analyzed time periods: Colletes chengtehensis, Dasypoda hirtipes, Halictus semitectus, Lasioglossum calceatum, L. discum, L. lucidulum, L. sexnotatum and Melitta tricincta, of which four species can be characterized as sand-affine (Colletes chengtehensis, Dasypoda hirtipes, Halictus semitectus, Lasioglossum lucidulum) and three as steppe-associated (Colletes chengtehensis, Halictus semitectus, Lasioglossum discum) species. Over the whole studied time period, 155 species (53.6%) were polylectic, 75 species (26%) oligolectic and 59 parasitic (20.4%), 47 species (16.3%) were sand-affine and 58 (20%) highly depend on steppe-like habitats.
Comparison of wild bee species richness and ecological traits across time periods
Across the two periods with approximately complete samples (1931–1966 vs. 2000–2021), 283 of the 289 species were recorded, with an overlap of 82 species, while 164 species occurred only in the earlier period, and 37 species only in the later one. The total species richness decreased with over 50% between the two periods, from 246 to 119 species. Taking all time periods into account, 28 species were recorded from the site for the first time after 2001.
Lecty
The data from the two periods with approximately complete samples showed an increase of oligolectic species from 23.5% (58 of 246 spp.) in 1931–1966 to 28.5% (34 of 119 spp.) in 2001–2021. This pattern was even more pronounced, comparing species documented only in 1931–1966 with a relative amount of 23.8% oligolecty, to species documented only in 2001–2021 with 40.5% oligolecty. Changes in lecty were similar across the four time periods, which include all wild bee data (Fig. 2A).
Parasitic species
The proportion of total parasitic species decreased from 22% (54 of 246 spp.) in 1931–1966 to 18.5% (22 of 119 spp.) in 2001–2021. Again, this pattern was even clearer comparing species that occurred only in 1931–1966 with 22.5% parasitic ones versus 13.5% in the species which occurred only in 2001–2021.
Nesting type
While 91.5% (174 of 190 spp.) of the species recorded in 1931–1966 were ground nesting (dig into soil for nest construction), the proportion decreased to 69.3% (67 of 97 spp.) in 2001–2021. Instead, the number of above-ground nesting species showed a reversed trend: Species nesting in wooden structures or shrubs increased from 6% (12 of 190 nest-building spp.) in 1931–1966 to 24% (24 of 97 nest-building spp.) in 2000–2021. Specifically, species nesting in dead wood substrates increased from 2.2 to 4.7%, species nesting in pre-existing cavities associated to tunnels created by wood-boring beetles increased from 3.8 to 8.2% and such species nesting in dead twigs or plant stems increased from 5.5 to 10.8%. The proportion of species nesting in snail shells increased from 1 to 3%. Changes in the proportion of species with different nesting type were similar across the four time periods (Fig. 2B).
Habitat requirements
The proportion of sand-affine species decreased from 18.3% (45 of 246 spp.) in 1931–1966 to 13.4% (16 of 199 spp.) in 2001–2021. Similarly, the proportion of steppe-associated species decreased from 21.1% (52 of 246 spp.) in 1931–1966 to 15.1% (18 of 119 spp.) in 2001–2021. The proportion of ubiquitous species increased slightly from 21.5% (53 of 246 spp.) in 1931–1966 to 22.7% (27 of 119 spp.) in 2001–2021.
Species adapted to open land habitats decreased from 54% (133 of 246 spp.) in 1931–1966 to 47% (56 of 119 spp.) in 2001–2021. Species requiring forest edges and related habitats increased relative to the absolute number of species recorded in the respective time period from 24.4% (60 of 246 spp.) in 1931–1966 to 30.3% (36 of 119 spp.) in 2001–2021. Looking specifically at the species recorded exclusively in one of the time periods, 54.9% open land species and 25% edge species were recorded in the time period 1931–1966 versus 35% open land species and 45.9% edge species in 2001–2021. The proportional changes of species with different habitat requirements were similar across the four time periods (Fig. 2C).
Flight period
Species with their main flight period in spring decreased from 30.9% (75 of 246 spp.) in 1931–1966 to 21% (25 of 119 spp.) in 2001–2021, while species mainly flying during summer increased from 33.33% (82 of 246 spp.) to 45.4% (54 of 119 spp.). Species with a later or long flight period did not change as much (2% vs. 3.4%, 33.7% vs. 30.3%). The proportional changes of species with different flight periods were similar across all time periods (Fig. 2D). The nesting preferences differ with the flight period, with 91.7% of all non-parasitic spring species being ground nesting (55 of 60 spp.), 70.5% of the summer species (62 of 88 spp.), 100% of the autumn species (6 spp.) and 90.7% of the species with a long flight period (69 of 76 spp.).
Land use change
In total 883,125 ha area per year (1966, 1994, 2000, 2018) was mapped from historical aerial photographs and orthophotos (Table 1). The landscape was dominated by agricultural areas across all periods (Fig. 3A–D). Grassland, fallows and woods with different proportions of canopy cover were characteristic semi-natural habitats in the study area (Fig. 3A–D).
While the total agricultural area in the study area remained relatively similar over time (Fig. 4A), the area covered with woods increased from 1966 (124.49 ha) to 2018 (174.75 ha). Specifically, areas characterized by medium and sparse wood cover increased, while woods with continuous canopy cover increased from 1966 to 1994, but later decreased between 2000 and 2018 (Fig. 4B; Table 1). Furthermore, the total area covered with grassland and fallows decreased slightly between 1966 and 1994 and stayed equal afterwards (Fig. 4B). The total area with anthropogenic entities such as villages, buildings or industrial sites increased steadily from 1966 to 2018.
Changes in wild bee community composition
The whole wild bee community (Fig. 5A; r2 = 0.66; p = 0.02) as well as the community including only sand and steppe specialists (Fig. 5C; p = 0.007) were significantly different among the four time periods. Vector fitting revealed that the bee community in the period 1967–2000 (Fig. 5A) was significantly associated with the area of closed woods (r2 = 0.78; p = 0.03), bare ground patches (r2 = 0.9; p = 0.001) and field margins (r2 = 0.93; p = 0.001). Across all time periods, the CWMs for lecty were significantly (r2 = 0.15; p = 0.023) associated with the NMDS (Fig. 5A). Results from vector fitting indicated on the one hand, that oligolectic species were characteristic for the late 1930s and early 1940s, but on the other hand oligolectic species may also have benefitted from the increasing area of field margins after 1966 (Fig. 5A).
Non-metric Multidimensional Scaling and significant (p ≤ 0.05) results from vector fitting of land use change (data fitted for period 1966 to 2021), and community weighted means of A the whole wild bee community, B oligolectic wild bee species and C wild bee species specialized on sand and steppe habitats in and around the nature preservation area in Oberweiden (Lower Austria) between 1882 and 2020. Ellipses show clustering of wild bee communities among different time periods. Land use categories: Anthr anthropogenic entity, BG bare ground patches, CW closed woods, DRwithV dirt road with central vegetation, DRnonV dirt road without central vegetation, FM field margin, GL grassland, MOW medium open wood, SW sparsely wooded. Traits: pl polylectic, ol oligolectic
When specifically analyzing the oligolectic bee community (Fig. 5B) the vector fitting revealed that dependence on different plant taxa were significantly associated with the wild bee community of the different time periods (r2 = 0.42; p = 0.045). In the two earlier periods the oligolectic species assemblage was specialized on Dipsacaceae, Brassicaceae and Fabaceae. Also, parasitic species depending on oligolectic hosts were associated with these periods. During the two later periods the oligolectic wild bee community was characterized by species specialized on Asteraceae or specifically on Carduoideae.
The wild bee community including only species depending on sand and steppe habitats (Fig. 5C) and for the most recent time period (2001–2021) was significantly associated with grassland (r2 = 0.78; p = 0.03), medium (r2 = 0.77; p = 0.04) and sparsely wooded areas (r2 = 0.78; p = 0.04) as well as anthropogenic entities (r2 = 0.78; p = 0.05). The sand and steppe bee community during the earlier time period (1967–2000) showed a tendential relation with closed woods (r2 = 0.77; p = 0.04) and bare ground patches (r2 = 0.79; p = 0.04).
Discussion
The results of our study indicate that changes that led to a major decline of species richness likely occurred during the first half of the twentieth century or even earlier: about half of the species documented from the site were last recorded in the 1950s or earlier, and many species were already rare at that time (Pittioni and Schmidt 1942, 1943). Furthermore, 14 of 33 species which are regionally extinct in Austria (Kratschmer et al. 2021) and endangered at the European level (Nieto et al. 2014), have been recorded from the site before 1966, with a high proportion adapted to steppe and sand habitats. Specifically, these are Amegilla quadrifasciata, Andrena hungarica, A. morio, A. transitoria, Bombus armeniacus, B. fragrans, B. laesus, Colletes albomaculatus, C. punctatus, Dasypoda braccata, D. suripes, Nomada melathoracica, Pseudapis femoralis and Eucera (Tetraloniella) pollinosa. Rare species, e.g. species with population sizes near their extinction threshold, are most likely to have an extinction debt (Hanski and Ovaskainen 2002; Kuussaari et al. 2009). The time delay between an environmental change and the disappearance of a species depends on the size and isolation of the habitat fragment, as well as the generation time of the species, but can be assumed to be tens of years (Kuussaari et al. 2009). Thus, the critical changes in the environment probably took place at the beginning of the twentieth century or maybe even at the end of the nineteenth century.
Similar trends have been documented in other European countries, for example Belgium, the Netherlands, Sweden and Great Britain, where a decline of wild bee species between the 1950s and 1990 has been observed, along with a biotic homogenization driven by an expansion of common bee species (Jauker et al. 2009; Bommarco et al. 2012; Carvalheiro et al. 2013). Also, in the northeastern US, native wild bee species richness declined steadily over the last 140 years (Bartomeus et al. 2013).
Apart from a dramatic decline of species richness since the period 1931–1966, we also found 28 species, which were only recorded in the most recent period. Among the newly recorded species, Ceratina nigrolabiata, Icteranthidium laterale, Lithurgus chrysurus, L. cornutus, Osmia bidentata, O. spinulosa, Pseudapis diversipes, and its parasite Pasites maculatus currently expand their distribution from warmer, more southern and eastern regions to Austria, probably as a response to climatic warming (Zettel et al. 2019b; Pachinger et al. 2019). However, it should be mentioned here, that climate change does not necessarily benefit wild bee communities, as recently demonstrated for alpine ecosystems, where dramatic changes in bumblebee communities were observed (Scharnhorst et al. 2023).
We have studied the changes in landscapes and found correlations with the changes in the bee community (Fig. 5). However, land use changes detectable on aerial images affect only a small portion of the entire area. Changes in land management (e.g. mowing regime, grazing, use as a horse track) might have had a greater impact on changes in the bee fauna, similar to the findings of Cousins et al. (2015) who describe the influence of the changing character of land use on biodiversity due to management changes. Such qualitative changes could not be considered in the statistical analysis, because of lacking data, but could have an impact on the interpretation of the results. If more detailed data on the land use (size of mowed patches, extent of grazing, use of fertilizers in the surrounding fields etc.) would have been available, these might have shown stronger correlations than the quantitative change in area size. Furthermore, aerial images were only available from 1966 onwards, thus the time prior to the last records of about half of the species documented from the site is not reflected.
Additionally, digitization of polygons from aerial photos/orthophotos has several limitations compared to field mapping, as structures and cover may not always be clearly detectable (e.g. agricultural field vs fallow). Further, picture quality differed between the years, which made assignability of land use categories sometimes difficult. For example, the 1966 aerial photo was only available in black and white, and the 1994 photo was available only in lower resolution than the ones from 2000 or 2018. Other studies summarized the methodological drawbacks of digitization procedures of historical maps and pictures, though concluding it is still the best way of gathering information of historical land use (Geri et al. 2010).
Shift from ground to above-ground nesting species
The decrease of the proportion of ground nesting species of over 20% (91.5% to 69.3%) shows a distinct shift towards above-ground nesting species since the 1930s (Fig. 2B). The shift from spring to summer species observed on the site (Fig. 2D) contrasts with the results of other recent studies showing increased extinction vulnerability of late-summer active bee species in Central Europe (Scheper et al. 2014; Hofmann et al. 2019). The shift observed in Oberweiden was likely correlated with the decrease of ground-nesters, as they make up a much higher proportion of the species active in the spring, compared with the summer species (91.7% vs. 70.5%). While no landscape data were available for the time period 1930–1966, changes in the bee community of 1967–2000 significantly correlated with changes in forest structures (Fig. 5A), specifically with ”closed wood”. This can be explained by increased loose tree structures (medium open wood, sparsely wooded) accompanying the decrease of closed wood area. Especially above-ground nesting bee species, which likewise proportionally increased in that time period (Fig. 2B), benefitted from open woody structures, as they provide a variety of nesting structures.
Despite measures to remove woody plants implemented in 2004 as part of an EU-LIFE project (Wiesbauer 2002b), the total area of open grassland is still smaller today than in 1966, while the sparsely wooded area increased (Fig. 4). Both, the decrease of grassland area and the increase of medium to sparsely wooded area significantly relate to the sand and steppe bee community in the time period 2001–2021 (Fig. 5C). Similar results were found in a study of several steppe-associated bee species in the Czech Republic. That study documented an increase in above-ground nesting bee species and a decrease in ground nesting species during 1990–2017 compared to 1930–1990 (Bogusch et al. 2020).
Besides the succession of grassland to sparsely wooded land, a successional shift from steppe grassland to a dense meadow with shrubs probably have affected the ground nesting bee community. The extent of bare ground patches, flower abundance and diversity, crucial elements for ground nesting bees, are often reduced in later successional stages through competitive exclusion by increasingly dominant grasses (Albrecht et al. 2021). Such changes might have been fostered by three major factors:
-
A.
The shift from traditional rotational grazing to mowing: Low-intensity grazing, as practiced in former times by pastured herds, was certainly more beneficial for the bee community than the homogenizing regime of the currently practiced large-scale mowing (Tonietto and Larkin 2018). Short time periods of intensive grazing create small-scale heterogeneity and bare ground patches through the trampling. Though localized eutrophication from defecation is possible, it contributes to nutrient removal in the long term, especially if the animals are kept in a barn during the night (Wiesbauer and Mazzucco 1997).
-
B.
Plantings of windbreaks: The formerly open sandy areas used to be regularly moved by strong winds in the area and accumulated as dunes (Wiesbauer 2002c). Wind erosion can have a positive effect on biodiversity by causing organic matter to decline and thus fostering plant species adapted to nutrient-poor environments (Riksen et al. 2006; Ödman and Olsson 2014). To make the land more suitable for agriculture, a close-meshed network of windbreaks has been planted since the eighteenth century, reducing the wind speed and thus promoting advanced succession of sandy grasslands (Wiesbauer 2002b). Such land-use changes counteracting extensive sand movement and drift have been understood as a key factor in the decline of xeric sandy calcareous grassland in Europe (Ödman and Olsson 2014).
-
C.
Nitrification has been documented to greatly influence plant and insect communities (WallisDeVries and van Swaay 2017; Wagner 2020; Raven and Wagner 2021), and is particularly likely to have affected the specialized steppe plant community of the site negatively by promoting fast-growing grasses and woody plants. It might be an effect of an introduction of nutrients from the environment (Bobbink et al. 1998) or related to decalcification as lime binds available nutrients (Ödman and Olsson 2014).
Loss of floral resources affects bee abundance and diversity
The amount of floral resources is a key factor driving wild bee diversity and its loss (Potts et al. 2003; Scheper et al. 2014). Besides the severe decline of species diversity, the decrease of the proportion of parasitic species is also an indicator of declining or highly fluctuating population sizes of host bee species (Sheffield et al. 2013) and may corroborate changes in the availability and reliability of floral resources. The management of the conservation area with mowing of almost the entire area in mid-June (Wiesbauer 2002b; pers. comm. M. Lödl and pers. obs.) might represent a major recent factor negatively affecting the quality of the habitat. Large scale mowing has a profound effect on bee populations, as it leads to an immediate and devastating decrease of floral resources needed for survival and reproduction.
Of particular interest when it comes to flowering recources are bee species specialized in the pollen of teasel family (Dipsacaceae), as they are particularly endangered in Europe (Nieto et al. 2014; Potts et al. 2015) and even may become the focus of a European Action Plan for pollinator conservation (Hochkirch et al. 2021). The vector fitting of CWMs of the oligolectic species and their specific host plants revealed a specialization on Dipsacaceae only in the two earlier periods. Two bee species in the focus of the European Action Plan are both endangered at the European level (Nieto et al. 2014), and regionally extinct in Austria, with the last records from 1940 in the study area (Kratschmer et al. 2021). Specifically, these are the dark pantaloon bee Dasypoda braccata, oligolectic on Scabiosa ochroleuca in Austria (Praz et al. 2008), and the swollen pantaloon bee Dasypoda suripes, specialized on Knautia arvensis and Scabiosa species. Likewise, the near threatened silvery pantaloon bee Dasypoda argentata, also specialized on S. ochroleuca, was observed at the site in 1959 for the last time in Austria (Online Resource 2). One reason for the disappearance of these bee species from the study area is likely a decrease of the abundance of their host plant species. These Dipsacaceae species must have been common in the study area previously, as a large number of flowers seems to be necessary for a population to survive. It has been calculated for Andrena hattorfiana, a bee species of comparable large body size, that a medium-sized population of 50 females needs pollen from 924 plants of K. arvensis to be self-sustaining (Larsson and Franzen 2007; Zurbuchen and Müller 2012). While still sparsely distributed plants of K. arvensis and S. ochroleuca occur in the area today (pers. obs.), their quantity is likely not enough to sustain viable populations of one or several relatively large bee species. A study of the reproductive success of S. ochroleuca under a future climatic scenario with drier summers and wetter springs and falls, showed an increased seed production in summer: The drought-tolerant species is more affected by competition than by water availability and in the contrary seems to benefit from a lower competitor biomass in drier summers (Andrzejak et al. 2022). A strong effect of competition on the abundance of S. ochroleuca has been shown under abandonment of grazing under varying densities of the grass Festuca rupicola (Partzsch et al. 2017). This suggests that the disappearance of bee species oligolectic on Dipsacaceae might be related with a qualitative successional shift from steppe grassland to a denser grass-dominated meadow.
Altogether, our study corroborates the bad conservation status of habitats and species of Pannonic sand steppes compared to the favourable status described in the Habitats Directive, as assessed in Austria in the evaluation 2013–2018 by the European Environment Agency (2022). Stronger reference to historic land management practices, such as short time periods of intensive grazing and small-scaled, staggered mowing would be desirable to improve the habitat quality. However, more drastic measures, such as removal of the topsoil in some parts and changes in the landscape to re-establish exposure to wind erosion, might be necessary to restore the area to its condition 100 years ago (Riksen et al. 2006; Ödman and Olsson 2014). Finally, a decoupling of the management of high priority conservation areas from the agricultural incentive system by establishing an independently funded, not profit-oriented organization dedicated to the conservation objective could reduce the risk of mismanagement. A good example are sites belonging to the Austrian non-profit organization Naturschutzbund, which are, however, entirely managed by volunteers due to underfunding (Naturschutzbund 2023).
Conclusions
In this study we aimed at identifying changes of the wild bee community of a protected steppe habitat in Eastern Austria over a period of over 100 years and correlating these with land use change recognized from aerial photographs since 1966. We found that the bee community strongly differed between the four defined time periods regarding species numbers, species composition and their ecological traits. About half of the wild bee species was only recorded before 1966, indicating that critical changes in the environment took place already at the beginning of the twentieth century. A distinct shift towards above-ground nesting species since the 1930s is explained by land management practices and changes in the landscape as plantings of windbreaks, promoting advanced succession of the site. Quantitative land use change since 1966 was subtler than expected, but did correlate with the bee community: particularly, the transition from grassland to sparsely and medium wooded areas correlated with sand and steppe specialists, and a dependence of the wild bee community on different plant taxa in the different time periods were highlighted.
References
Albrecht M, Knecht A, Riesen M, Rutz T, Ganser D (2021) Time since establishment drives bee and hoverfly diversity, abundance of crop-pollinating bees and aphidophagous hoverflies in perennial wildflower strips. Basic Appl Ecol 57:102–114. https://doi.org/10.1016/j.baae.2021.10.003
Amiet F, Müller A, Neumayer R (1999) Apidae 2—Colletes, Dufourea, Hylaeus, Nomia, Nomioides, Rhophitoides, Rophites, Sphecodes, Systropha. Fauna Helvetica 4, CSCF & SEG, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumayer R (2001) Apidae 3—Halictus, Lasioglossum. Fauna Helvetica 4, CSCF & SEG, Neuchâtel
Andrzejak M, Korell L, Auge H, Knight TM (2022) Effects of climate change and pollen supplementation on the reproductive success of two grassland plant species. Ecol Evol 12:e8501. https://doi.org/10.1002/ece3.8501
Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R (2013) Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc Natl Acad Sci 110:4656–4660
Bobbink R, Hornung M, Roelofs JG (1998) The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J Ecol 86:717–738
Bogusch P, Hlaváčková L, Šilhán K, Horsák M (2020) Long-term changes of steppe-associated wild bees differ between shell-nesting and ground-nesting species. J Insect Conserv 24:513–523
Bommarco R, Lundin O, Smith H, Rundlöf M (2012) Drastic historic shifts in bumble-bee community composition in Sweden. P Roy Soc B 279:309–315
Carvalheiro LG, Kunin WE, Keil P et al (2013) Species richness declines and biotic homogenization have slowed down for NW-European pollinators and plants. Ecol Lett 16:870–878. https://doi.org/10.1111/ele.12121
Carvell C (2002) Habitat use and conservation of bumblebees (Bombus spp.) under different grassland management regimes. Biol Conserv 103:33–49
Carvell C, Meek WR, Pywell RF, Nowakowski M (2004) The response of bumblebees to successional change in newly created arable field margins. Biol Conserv 118:327–339
Cousins SAO, Auffret AG, Lindgren J et al (2015) Regional-scale land-cover change during the 20th century and its consequences for biodiversity. Ambio 44:17–27. https://doi.org/10.1007/s13280-014-0585-9
Dathe H (1980) Die Arten der Gattung Hylaeus F. in Europa (Hymenoptera: Apoidea, Colletidae). Mitt Zool Mus Berl 56:207–294
Dathe H, Scheuchl E, Ockermüller E (2016) Illustrierte Bestimmungs-tabelle für die Arten der Gattung Hylaeus F. (Maskenbienen) in Deutschland, Österreich und der Schweiz. Entomologica Austriaca Suppl 1:1–51
Dengler J, Janišová M, Török P, Wellstein C (2014) Biodiversity of Palaearctic grasslands: a synthesis. Agric Ecosyst Environ 182:1–14. https://doi.org/10.1016/j.agee.2013.12.015
Dorchin A, López-Uribe MM, Praz CJ, Griswold TS, Danforth BN (2018) Phylogeny, new generic-level classification, and historical biogeography of the Eucera complex (Hymenoptera: Apidae). Mol Phylogenet Evol 119:81–92. https://doi.org/10.1016/j.ympev.2017.10.007
Dornelas M, Antão LH, Moyes F et al (2018) BioTIME: a database of biodiversity time series for the Anthropocene. Glob Ecol Biogeogr 27(7):760–786. https://doi.org/10.1111/geb.12729
Dornelas M, Daskalova GN (2020) Nuanced changes in insect abundance. Science 368:368-369F
Ebmer AW (1969) Die Bienen des Genus Halictus Latr. s. l. im Großraum von Linz (Hymenoptera, Apidae) Teil 1. Naturkundliches Jahrbuch Der Stadt Linz 15:133–183
Ebmer AW (1970) Die Bienen des Genus Halictus Latr. s. l. im Großraum von Linz (Hymenoptera, Apidae) Teil 2. Naturkundliches Jahrbuch Der Stadt Linz 16:19–82
Ebmer AW (1971) Die Bienen des Genus Halictus Latr. s. l. im Großraum von Linz (Hymenoptera, Apidae) Teil 3. Naturkundliches Jahrbuch Der Stadt Linz 17:63–156
Ebmer AW (1973) Die Bienen des Genus Halictus Latr. s. l. im Großraum von Linz (Hymenoptera, Apoidea) Nachtrag und zweiter Anhang. Naturkundliches Jahrbuch Der Stadt Linz 19:123–158
ESRI (2021) ArcGIS Pro 2.8.0. Environmental Systems Research Institute, Redlands
European Council (2007) Annex I: natural habitat types of community interest whose conservation requires the designation of special areas of conservation. https://eunis.eea.europa.eu/references/2324/habitats. Accessed 22 July 2022
European Environment Agency (2022) Pannonic sand steppes. https://eunis.eea.europa.eu/habitats/10125. Accessed 25 Mar 2023
Funnell J (2022) The Pittioni Bee Collection—digitized index. https://pittioni.myspecies.info/. Accessed 20 Dec 2021
GBIF (2022) The global biodiversity information facility. What is GBIF?. https://www.gbif.org/what-is-gbif. Accessed 22 Feb 2022
Geri F, Rocchini D, Chiarucci A (2010) Landscape metrics and topographical determinants of large-scale forest dynamics in a Mediterranean landscape. Landsc Urban Plan 95:46–53. https://doi.org/10.1016/j.landurbplan.2009.12.001
Goulson D, Nicholls E, Botias C, Rotheray E (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flower. Science 347:6229. https://doi.org/10.1126/science.1255957
Grüner Bericht (2022) Die Situation der österreichischen Land- und Forstwirtschaft. Bundesministerium für Land- und Forstwirtschaft, Regionen und Wasserwirtschaft, Wien
Hanski I, Ovaskainen O (2002) Extinction debt at extinction threshold. Conserv Biol 16:666–673
Hochkirch A, Vujić A, Flinn G (2021) Species action plans for EU pollinators—shortlist of 15 species action plans. Action plans for conservation of threatened pollinator species in the EU contract 07.0202/2020/839411/SER/ENV.0.2
Hofmann MM, Zohner CM, Renner SS (2019) Narrow habitat breadth and late-summer emergence increases extinction vulnerability in Central European bees. Proc R Soc B 286:20190316. https://doi.org/10.1098/rspb.2019.0316
IPBES (2016) The assessment report of the intergovernmental science-policy platform on biodiversity and ecosystem services on pollinators, pollination and food production. In: Potts SG, Imperatriz-Fonseca VL, Ngo HT (eds) Secretariat of the intergovernmental science-policy platform on biodiversity and ecosystem services, Bonn
Jauker F, Diekoetter T, Schwarzbach F, Wolters V (2009) Pollinator dispersal in an agricultural matrix: opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landsc Ecol 24:547–555
Kasy F (1957) Die Sandsteppe bei Oberweiden im Marchfeld—ein schutzbedürftiges Refugium östlicher Steppenarten in der Nähe Wiens. Natur und Land 43(5):61–64
Kennedy CM, Lonsdorf E, Neel MC, Williams NM, Ricketts TH, Winfree R et al (2013) A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett 16:584–599
Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B 274:303–313. https://doi.org/10.1098/rspb.2006.3721
Kratschmer S, Zettel H, Ockermüller E, Zimmermann D, Schoder S, Neumayer J, Gusenleitner F, Zenz K, Mazzucco K, Ebmer AW, Kuhlmann M (2021) Threat ahead? An experts’ opinion on the need for red lists of bees to mitigate accelerating extinction risks—the case of Austria. Bee World 98(3):74–77. https://doi.org/10.1080/0005772X.2021.1940734
Kuussaari M, Bommarco R, Heikkinen RK, Helm A, Krauss J, Lindborg R et al (2009) Extinction debt: a challenge for biodiversity conservation. Trends Ecol Evol 24:564–571
Küster H (1999) Geschichte der Landschaft in Mitteleuropa. CH Beck, München
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Laliberté E, Legendre P, Shipley B (2014) FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12.1
Larsson M, Franzen M (2007) Critical resource levels of pollen for the declining bee Andrena hattorfiana (Hymenoptera, Andrenidae). Biol Conserv 134:405–414
Naturschutzbund (2023) Mithelfen & mitarbeiten beim naturschutzbund. https://naturschutzbund.at/mithelfen.html Accessed 29 Mar 2023
Nieto A, Roberts SP, Kemp J, Rasmont P, Kuhlmann M et al (2014) European red list of bees. Publication Office of the European Union. https://doi.org/10.2779/77003
Ödman AM, Olsson PA (2014) Conservation of sandy calcareous grassland: what can be learned from the land use history? PLoS One:e90998. https://doi.org/10.1371/journal.pone.0090998
Oksanen J (2013) Vegan: ecological diversity. R Project 368:1–11
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: community ecology package. R package version 2.5–7.
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
Pachinger B, Kratschmer S, Ockermüller E, Neumayer J (2019) Notizen zum Vorkommen und zur Ausbreitung ausgewählter Wildbienenarten (Hymenoptera: Anthophila) in den Agrarräumen Ost-Österreichs. Beiträge Zur Entomofaunistik 20:177–198
Partzsch M, Faulhaber M, Meier T (2017) The effect of the dominant grass Festuca rupicola on the establishment of rare forbs in semi-dry grasslands. Folia Geobot 53:103–113. https://doi.org/10.1007/s12224-017-9298-8
Pittioni B, Schmidt R (1942) Die Bienen des südöstlichen Niederdonau I. Apidae, Podaliriidae. Xylocopidae und Ceratinidae Niederdonau/natur und Kultur 19:1–59
Pittioni B, Schmidt R (1943) Die Bienen des südöstlichen Niederdonau II. Andrenidae und isoliert stehende Gattungen. Niederdonau/natur und Kultur 24:1–89
Potts SG, Vulliamy B, Dafni A, Ne’eman G, Willmer P (2003) Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84:2628–2642. https://doi.org/10.1890/02-0136
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Potts S, Biesmeijer K, Bommarco R et al (2015) Status and trends of European pollinators. Key findings of the STEP project. Pensoft Publishers, Sofia
Praz C, Carron G, Michez D (2008) Dasypoda braccata Eversmann (Hymenoptera, Dasypodaidae), nouvelle espèce pour l’apidofaune italienne. Osmia 2:16–20
Praz C, Genoud D, Vaucher K, Bénon D, Monks J, Wood TJ (2022) Unexpected levels of cryptic diversity in European bees of the genus Andrena subgenus Taeniandrena (Hymenoptera, Andrenidae): implications for conservation. J Hymenopt Res 91:375–428. https://doi.org/10.3897/jhr.91.82761
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rabitsch W (2002) Die Wanzenfauna (Heteroptera) der Sandberge bei Oberweiden im Marchfeld (Niederösterreich). Beiträge Zur Entomofaunistik 3:141–174
Raven PH, Wagner DL (2021) Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc Natl Acad Sci USA 118:e2002548117. https://doi.org/10.1073/pnas.2002548117
Riksen M, Ketner-Oostra R, van Turnhout C, Nijssen M, Goossens D, Jungerius PD, Spaan W (2006) Will we lose the last active inland drift sands of Western Europe? The origin and development of the inland drift-sand ecotype in the Netherlands. Landsc Ecol 21:431–447
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Scharnhorst VS, Thierolf K, Neumayer J, Becsi B, Formayer H, Lanner J, Ockermüller E, Mirwald A, König B, Kriechbaum M, Meimberg H, Meyer P, Rupprecht C, Pachinger B (2023) Changes in community composition and functional traits of bumblebees in an alpine ecosystem relate to climate warming. Biology 12:316. https://doi.org/10.3390/biology12020316
Scheper J, Reemer M, van Kats R, Ozinga WA, van der Linden GTJ, Schaminée JHJ, Siepel H, Kleijn D (2014) Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc Natl Acad Sci USA 111:17552–17557. https://doi.org/10.1073/pnas.1412973111
Scheuchl E (1995) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, Band I: Anthophoridae. Self-published by Erwin Scheuchl, Velden
Scheuchl E (1996) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, Band II: Megachilidae – Melittidae. Self-published by Erwin Scheuchl, Velden
Scheuchl E, Willner W (2016) Taschenlexikon der Wildbienen Mitteleuropas—Alle Arten im Porträt. Quelle & Meyer Verlag, Wiebelsheim
Schmid-Egger C, Scheuchl E (1997) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs unter Berücksichtigung der Arten der Schweiz, Band III: Andrenidae. Self-published by Erwin Scheuchl, Velden
Sheffield CS, Pindar A, Packer L, Kevan PG (2013) The potential of cleptoparasitic bees as indicator taxa for assessing bee communities. Apidologie 44:501–510. https://doi.org/10.1007/s13592-013-0200-2
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83:1421–1432
Straka J, Bogusch P (2011) Contribution to the taxonomy of the Hylaeus gibbus species group in Europe (Hymenoptera, Apoidea and Colletidae). Zootaxa 2932:51–67
Tonietto RK, Larkin DJ (2018) Habitat restoration benefits wild bees: a meta-analysis. J Appl Ecol 55:582–590
Vanbergen AJ, Garratt MP, Baude M, Biesmeijer JC, Britton NF, Brown MJF et al (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11:251–259. https://doi.org/10.1890/120126
Vrahnakis MS, Janišová M, Rūsiņa S, Török P, Venn S, Dengler J (2013) The European Dry Grassland Group (EDGG): stewarding Europe’s most diverse habitat type. In: Baumbach H, Pfützenreuter S (ed) Steppenlebensräume Europas—Gefährdung, Erhaltungsmaßnahmen und Schutz, Thüringer Ministerium für Landwirtschaft, Forsten, Umwelt und Naturschutz, Erfurt, pp 417–434
Wagner DL (2020) Insect declines in the anthropocene. Annu Rev Entomol 65:457–480. https://doi.org/10.1146/annurev-ento-011019-025151
WallisDeVries MF, Van Swaay CA (2009) Grasslands as habitats for butterflies in Europe. In: Veen P et al (eds) Grasslands in Europe. KNNV Publishing, Zeist, pp 26–34. https://doi.org/10.1163/9789004278103_004
WallisDeVries MF, van Swaay CA (2017) A nitrogen index to track changes in butterfly species assemblages under nitrogen deposition. Biol Conserv 212:448–453. https://doi.org/10.1016/j.biocon.2016.11.029
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wiesbauer H (2002a) Dünen- und Flugsandgebiete in Niederöster-reich. In: Wiesbauer H (ed) Naturkundliche Bedeutung und Schutz ausgewählter Sandlebensräume in Niederösterreich. Bericht zum LIFE-Projekt „Pannonische Sanddünen“. Amt der NÖ Landes-regierung/Abteilung Naturschutz, St. Pölten, pp 7–14
Wiesbauer H (2002b) Pflegepläne für die Schwerpunktgebiete des LIFE-Projektes. In: Wiesbauer H (ed) Naturkundliche Bedeutung und Schutz ausgewählter Sandlebensräume in Niederösterreich. Bericht zum LIFE-Projekt „Pannonische Sanddünen“. Amt der NÖ Landesregierung/Abteilung Naturschutz, St. Pölten, pp 144–171
Wiesbauer H (2002c) Die Niederösterreichische Steppe—Bilder aus der vergangenen Tagen. Publikationen Naturschutzabteilung Niederösterreich 2:1–16
Wiesbauer H (2008) Die Steppe lebt. Felssteppen und Trockenrasen in Niederösterreich. Amt der NÖ Landesregierung, St. Pölten
Wiesbauer H (2020) Wilde Bienen. Biologie, Lebensraumdynamik und Gefährdung. Artenporträts von über 470 Wildbienen Mitteleuropas. Ulmer Verlag, 2nd edn, Stuttgart
Wiesbauer H, Mazzucco K (1997) Dünen in Niederösterreich—Ökologie und Kulturgeschichte eines bemerkenswerten Landschaftselementes. Fachberichte Des Niederösterreichischen Landschaftsfonds 6:1–90
Williams NM, Crone EE, T’ai HR, Minckley RL, Packer L, Potts SG (2010) Ecological and life-history traits predict bee species responses to environmental disturbances. Biol Conserv 143:2280–2291
Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90:2068–2076
Zattara EE, Aizen MA (2021) Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4:114–123
Zettel H, Zenz K, Kuhlmann M (2019a) Zur Verbreitung der Seidenbienenarten Colletes marginatus Smith, 1846 und Colletes chengtehensis Yasumatsu, 1935 in Österreich (Hymenoptera: Apidae: Colletinae). Entomologica Austriaca 26:7–24
Zettel H, Wiesbauer H, Schoder S, Hoffmann F (2019b) Zur Kenntnis der Wildbienen (Hymenoptera: Apidae) in Wien, Niederösterreich und dem Burgenland (Österreich)—9. Beiträge Zur Entomofaunistik 20:3–20
ZOBODAT (2021) Occurrences. https://www.zobodat.at/belege.php. Accessed 20 Dec 2021
Zurbuchen A, Müller A (2012) Wildbienenschutz—von der Wissenschaft zur Praxis. Haupt Verlag, Bern
Acknowledgements
We cordially thank Bärbel Pachinger (University of Natural Resources and Life Sciences, Vienna) and Johann Neumayer (Salzburg) for the contribution of historical records, Fritz Gusenleitner for the determination of Andrena species, and Esther Ockermüller for the determination of some Nomada species. We thank Sabine Gaal-Haszler for linguistically correcting the manuscript. We are most grateful to Niklas Johansson, one anonymous reviewer and Tobias Schernhammer whose constructive comments greatly helped to improve the manuscript. The survey of the bee species in the years 2018 and 2019 by DZ and SS was funded by the initiative “Give bees a chance” (Arcotel Hotels).
Funding
The survey of the bee species in the years 2018 and 2019 by DZ and SS was funded by the initiative “Give bees a chance” (Arcotel Hotels). The respective collecting permits are deposited at the NHMW.
Author information
Authors and Affiliations
Contributions
ZD and KS contributed to the study conception and design. Collection, preparation, and determination of the bees in the recent survey (2018–2019) was performed mainly by SS, and to a minor extent by ZD. ZH contributed with recent bee records from his collection and checked determinations of species which were difficult to determine. H-RC and KS mapped and analyzed the landscape data and KS did the statistical analyses. The manuscript was written by ZD (introduction, discussion, bee data) and KS (landscape data and analyses), and all authors commented on the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare. The authors have no relevant financial or non-financial interests to disclose. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Ethical approval
No ethics approval is required to study bees.
Consent for publication
The authors consent to participate in the review process and to transfer the copyright if the manuscript is accepted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dominique, Z., Sabine, S., Herbert, Z. et al. Changes in the wild bee community (Hymenoptera: Apoidea) over 100 years in relation to land use: a case study in a protected steppe habitat in Eastern Austria. J Insect Conserv 27, 625–641 (2023). https://doi.org/10.1007/s10841-023-00486-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00486-8