Abstract
Background
Euphydryas aurinia is a declining butterfly inhabiting oligotrophic grasslands in Central and Western Europe. Despite numerous ecological studies, patterns of its adult activity have so far been rather neglected, although adult resource use contributes to resource-based understanding of insects’ habitats.
Aim
To relate E. aurinia adult activity patterns to within-habitat vegetation structures.
Methods
(1) Timed adult activity observations along a transect crossing a colony site, analysed via partial ordination methods. (2) Activity records obtained during mark-recapture, analysed via binomial regressions.
Results
Both methods, besides influences of weather, time of day (similarities between morning and late afternoon hours), and progression of season (mate locating replaced by maintenance activities), revealed consistent association of behaviours to vegetation structures. Of the two male mate-locating behaviours, perching occurred near shrubs and woodland edges, and patrolling over centres of inhabited meadows. Female activity concentrated in nectar-rich mid-height sward near host plants. Consequently, male and female activity were partly spatially separated.
Implications for conservation
A habitat for E. aurinia should provide resources for all its activities in close proximity. Grasslands containing host plants should be dissected by structures such as shrubs, woodlot edges, or taller herbaceous vegetation, emphasising the importance of landscape heterogeneity for insect fauna.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preserving suitable habitats is the crucial condition for efficient conservation of invertebrates (Samways 2007) including butterflies (Settele et al. 2009; Warren et al. 2021). Increasingly detailed information on habitat requirements of increasing numbers of specialist butterflies (e.g. Kivinen et al. 2008; Turlure et al. 2011; Maes et al. 2014; Vrba et al. 2021) indicate that a habitat patch must contain all vital resources for diverse activities of the given species, including larval host plant and shelter, overwintering substrate, adult food and shelter, and substrates utilised for mate locating and copulation (Dennis 2010; Turlure et al. 2019). Such resources may exist syntopically (cf. Courtney and Duggan 1983) or disjunctly (cf. McKay 1991) in time and space, but they must be present within routine individual movements’ distances of the species/developmental stages concerned (Dennis 2010). Consequently, the habitat of a butterfly species should not be viewed merely as a “vegetation community” containing the species’ larval host plant, or as a land cover category recognised, e.g., by geographers or landscape planners (Vanreusel and Van Dyck 2007). This distinction is crucial for managing reserves, biological restoration, and recovery programmes. For instance, the finding that behavioural responses to vegetation structure affect the reproductive fitness of individuals, quite accepted in vertebrate conservation (Caro 2007), implies that managing vegetation structure may enhance, or suppress, local populations of rare and endangered species (Shreeve and Dennis 2011; Turlure et al. 2011).
Euphydryas aurinia (Rottemburg, 1775; Nymphalidae: Nymphalinae) is a much-studied butterfly declining across Western and Central Europe and protected by the EU Habitat directive (van Swaay et al. 2012). It forms multiple genetic lineages across its large Palaearctic range (Tolman and Lewington 2009; Junker et al. 2015; Korb et al. 2016), utilises multiple larval host plants (Singer et al. 2002; Meister et al. 2015; Ghidotti et al. 2018), and inhabits diverse biotopes, from Mediterranean xeric scrub (Munguira et al. 1997; Junker and Schmitt 2010) to subalpine meadows (Junker et al. 2010). In Western and Central Europe, it mainly inhabits humid oligotrophic grasslands (Hula et al. 2004; Bulman et al. 2007; Pielech et al. 2017; Junker et al. 2021). Threats by habitat loss are augmented by gregarious larval development, linked to prominent abundance fluctuations (Schtickzelle et al. 2005; Bulman et al. 2007; Botham et al. 2011; Zimmermann et al. 2011a; Johansson et al. 2020), and vulnerability to inappropriate vegetation management, such as uniform cuts of the occupied meadows in autumn (Hula et al. 2004) or too intensive grazing (Johansson et al. 2019). In functioning metapopulation systems, local extinctions are compensated by a good dispersal ability, allowing recolonisation of sites over 10 km apart (Warren 1994; Zimmermann et al. 2011b; Junker et al. 2021).
So far, studies targeting E. aurinia focused mainly on consequences of its metapopulation dynamics, i.e., modelling of colonisation/extinction probability (Wahlberg et al. 2002; Schtickzelle et al. 2005; Bulman et al. 2007; Zimmermann et al. 2011b), genetic population structure (Wang et al. 2003; Sigaard et al. 2008; Davis et al. 2021; Junker et al. 2021; Pertoldi et al. 2021), and larval habitat requirements (Anthes et al. 2003; Tjørnløv et al. 2015, Psechera and Warren 2018). The patterns of habitat use by adults remain rather neglected, despite existing evidence that the structuring of adult habitats influences multiple vital aspects of many Nymphalidae (e.g., Janz 2005; Swartz et al. 2015; Sielezniew et al. 2019; Vrba et al. 2021), including representatives of the Euphydryas genus. (Murphy et al. 1984; Bennett et al. 2014; Pennekamp et al. 2014).
Here, we explore the relations between vegetation structure and adult E. aurinia activity on humid grasslands in Western Bohemia, Czech Republic. For several years, we surveyed the population using mark-recapture (Zimmermann et al. 2011a, b) supplemented by other methods targeting the species’ conservation requirements. This paper analyses two types of evidence: (a) observation of the butterfly activity, obtained during repeated timed walks along a fixed transect crossing a colony site; and (b) records of behaviour and substrate prior to the captures, recorded during marking the butterfly. Using these two approaches, we investigate adult temporal behavioural patterns and relationships between adult activity and structural features of its habitat. We predict that distinct behaviours will be associated with distinct habitat structures and that the association will be detectable despite activity changes due to weather conditions, time of day, and progressing season.
Material and methods
Study system
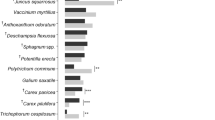
Field data originated from a network of humid seminatural grasslands near Karlovy Vary, Western Czech Republic (50° 9′ N, 13° 2′ E, altitude 650 m), on a hilly piedmont of the volcanic Doupovské Mts. We worked within a network of 28 ha humid meadow patches, separated one from another by ponds, shrubby hedges, and woodlots. In terms of vegetation, they are classified as intermittently wet Molinia meadows (association Junco effusi-Molinietum caeruleae Tüxen 1954; Fig. 1). The wider area represents a regional stronghold for humid grassland butterflies (Fric et al. 2010).
The population of Euphydryas aurinia develops solely on Succisa pratensis (Moench, 1794) on which the larvae feed gregariously until hibernation and solitarily in the spring. The adults emerge in late May and the emergence is protandrous, ♀♀ appear a few days after ♂♂. The flight period lasts about three weeks (Zimmermann et al. 2011a). While ♀♀ split their time between nectaring and egg laying, ♂♂ invest much time into mate-locating activities. Notably, they use two distinct mate-locating tactics, perching and patrolling (Wahlberg 2000; Wahlberg et al. 2001). The same ♂♂ individuals can alternate these two activities during their lifetime (unpublished data).
Transect observation of adult activity
In 2003, concurrently with marking the butterflies (carried out 28 May–17 June, cf. Zimmermann et al. 2011a), we set a fixed transect (total length: 970 m), crossing the meadows inhabited by E. aurinia. It was divided into 15 sections (mean length: 63 m ± 32.2 SD, range 20–150 m), separated by distinct landmarks and characterised by vegetation physiognomy (Fig. 1).
For eight days spread across the flight period (30–31 May; 2, 5, 7–10 June), we repeatedly walked the transect between 8:30 a.m. and 17:30 p.m. (CEST), usually twice per hour but with some variation due to weather, summing up to 132 walks (mean per day: 16 ± 1.3 SD, range 12–18). During the walks, we recorded all E. aurinia individuals seen per walk and section as in standard Pollard transects (Pollard and Yates 1993) but considering a smaller distance to the recorder (an imaginary 3 × 3 × 3 m cube), so that it was possible to record their behaviour.
The behaviours recorded, modified to fit the situation in E. aurinia, were, in ♂♂: Basking, Flight (direct, uninterrupted), Patrolling (gliding low over vegetation), Perching (settled at a prominent landmark, such as a tall grass blade or overhanging tree branch, at a sunny spot, ready for take-off), Chasing (with another butterfly/insect), Mating, Nectaring, and Resting (wings closed, hidden position, usually in shade). In ♀♀, we recognised Basking, Flight, Oviposition, Chasing, Mating, Nectaring, and Resting.
Each walk was characterised by date, time of day (i.e., closest hour), and visit to each section by a set of predictors, which could vary with individual walks. (A) Weather: Sky, describing overall cloudiness, 1: overcast, 2: half-cloudy, 3: clear; Sun, describing the momentary insolation of the given section, 1: fully shaded, 2: partly shaded, 3: fully sunlit; Wind, ranked on a 1–4 scale, from none to strong; and Dew, 1 standing for wet and 0 for dry sward. (B) Habitat structures: Host plant: estimated visually, 1: absent, 2: a few scattered rosettes, 3: even distribution, or a few clumps along the section, 4: monodominant clumps covering > 10 m2. Nectar: a ranked scale, 1: no flowers, 2: some flowers, 3: richly flowered. Height of the sward, estimated as % of the given section and differentiating Low sward: < 25 cm; Mid-height sward: < 50 cm; 3: Tall sward: > 50 cm. Shrubs: estimated visually, 1: none, 2: a few small and short solitary shrubs, each with projected ground cover < 4 m2, 3: larger clumps with projected cover > 4 m2, but shorter than 3 m; 4: large rows of scrub taller than 3 m or high forest edges.
We analysed the data using canonical correspondence analysis (CCA), a unimodal ordination technique relating the composition of samples to external predictors, in CANOCO v.5 (Ter Braak and Smilauer 2012). Each section walk represented a sample, and the activity records were the multivariate dependent variables. Significances of the ordinations were tested using 999 Monte-Carlo permutations, accounting for the spatial (consecutive sections of the transect) and temporal (repeated walks) structure in the data by using a split-plot design, permuting the data as cyclic shifts on both whole-plot and split-plot levels. Because this permutation design does not allow “empty” samples, a small number (0.001) was added to each cell in the response data table.
Targeting the response of activities to vegetation, we first controlled for nuisance effects of transect length, hour, and serial day (cf. Vlasanek et al. 2018). We selected the best-fitting response to hour and day from linear, polynomial, and factorial codings, and used the coding explaining the highest amount of variation in response data (Var%). Next, we assessed the response to weather. Finally, we constructed a covariate model containing hours, day, and weather variables selected by forward selection, and vegetation variables as predictors.
Activity records from mark-recapture
MR data originated from 2002 (24 May–28 June, 1141 behavioural records), 2003 (28 May–17 June, 2852 records), and 2004 (27 May–15 July, 2642 records). The marking was realized in a standard way: the butterflies were netted, marked with unique codes using alcohol-based pens, and released at points of capture. For every handling event, we also recorded the individual’s sex, time of capture (closest hour), weather (Sky and Wind, using the same system in the transect walks), and the butterfly’s behaviour prior to capture.
As above, we distinguished Resting, Basking, Nectaring, Flight, Patrolling, Reproduction (mating in ♂♂, mating plus oviposition in ♀♀), and Chasing. In addition, we recorded if the activity occurred at meadow edge (two-level categorical predictor), defined as located within approximately 3 m distance perpendicularly from a contiguous vertical wall of trees or shrubs, and near a host plant (again two categories, yes or no), again defined as approximately 3 m apart from the closest host plant. Edge data were recorded in all three years, host plant data only in 2003 and 2004.
Regression models relating the occurrence of the activity patterns to habitat edge or host plant were constructed using the glm function with binomial distribution in R 3.6.2 (package stats, family “binomial”, link function “logit”). For each type of behaviour, separately for ♂♂ and ♀♀ and for each of the three years, the modelling followed the same routine. We first entered variables describing weather during the individual observation, influencing butterfly activity considerably and rapidly. Second, we entered the hour in linear or quadratic forms to account for systematic effects of diurnal activity rhythms. Third, we entered the effect of serial day, again in linear and quadratic form, to account for possible seasonal effects. We used ΔAIC (≤ 2.0) to decide which variables to retain in the respective model. To the thus constructed covariate models, we sequentially added edge (all three years) and host plant (2003 and 2004 only) effects, again using AIC-statistics to decide whether either of these two predictors, or their combination, improved the fit of the model in question.
Results
Transect monitoring of butterfly activity
We obtained 2194 ♂♂ and 376 ♀♀ activity records. The most frequent ♂♂ activities were chasing (598), perching (546), patrolling (552) and nectaring (323); those of ♀♀ were nectaring (118), flight (107), basking (79) and oviposition (39); there were 23 records of mating.
In the CCA analyses, all potential covariables influenced the distribution of records significantly (Table 1), although there were notable differences. Section length was a weak predictor, indicating that other circumstances were much more important. Section identity, in contrast, was the strongest of all predictors, clearly because the sections differed in vegetation structures, and butterfly activity reflected this. For weather (Fig. 2a), the distinction Sun plus Sky versus Dew represented the main gradient. In both sexes, basking, resting, and (less so) nectaring were positively associated with wet sward, low Sun and overcast Sky, while the opposite applied for chasing, ♂♂ patrolling and perching. On the secondary ordination gradient, resting of both sexes was often observed in windy conditions. Hour fitted the data best if coded as a category (Fig. 2b). The main gradient of variation was between mornings plus late afternoons, when both sexes mainly rested or nectared; and mid-days, when chasing, ♀♀ oviposition, and ♂♂ patrolling and perching peaked. Mating peaked in the afternoon hours. Ordination with day (Fig. 2c), again best coded as factor, revealed a difference between early and late flight period, with ♂♂ prevailing in early and ♀♀ in late season. For ♂♂, the peak of patrolling seasonally preceded those of perching and nectaring; while for ♀♀, the peak of mating preceded that of nectaring and oviposition.
CCA ordination biplots (first—vertical and second—horizontal axes) showing Euphydryas aurinia activity records collected along a fixed transect route across a colony site (cf. Fig. 1). Black triangles are males, empty diamonds are females. a Effects of Weather, with section length as covariate (~ Weather | Length). b Effects of categorical Hour, with length and weather as covariates (~ Hour | Length + Sun + Sky + Dew). c Effects of categorial Day, with length and weather as covariates (~ Day | Length + Sun + Sky + Dew). d Effects of Vegetation structures, with forward-selected covariates (~ Vegetation | Day + Hour + Length + Wind + Sky + Dew). See Table 1 for relevant statistics of the models
Vegetation alone explained second-highest proportion of variation in the behavioural records after section identity, and retained its significant effect if controlled for hour, day, and weather, and even section, and in a complex covariate model (Table 1). The ordination diagrams, both without control for covariates (not shown) and after filtering their effects (Fig. 2d) showed that the first ordination gradient distinguished between mid- to tall sward with rich nectar and a high host plant representation, and conditions with shrubs and short sward. The tertiary gradient (not shown) distinguished short sward from mid- and tall sward. ♀♀ oviposited, basked, nectared, and rested near the host plants, though resting occurred in taller sward than the other activities. ♂♂ rested most frequently in tall sward near shrubs, perched and chased other insects near shrubs at shorter sward, and patrolled, nectared, and basked at short to medium sward, independently of host plant abundance. It follows that some ♂♂ activities, especially those associated with mate locating, were spatially separated from spots with high host plant cover, where ♀♀ performed most of their activities.
Activity records from mark-recapture
We obtained a total of 1251 behaviour records for 2002 (♂♂/♀♀: 876/375), 1624 for 2003 (1060/564), and 2639 for 2004 (2400/239). Across years, the most frequently recorded ♂♂ activities were patrolling (n = 1290), followed by perching (969), chasing (593), nectaring (495), basking (415), flight (396), resting (116), and reproductive activities (62). In ♀♀, the ordering was flight (414), basking (336), nectaring (259), resting (99), and reproductive behaviours (88). The higher number of reproductive behaviours, the majority of which concerned pairs marked in copula, was due to ♀♀ captured and marked during oviposition.
Results of the binomial regressions (Tables 2, 3), despite some inconsistencies among years, agreed with the established knowledge of adult butterfly activities. For instance, ♂♂ basking decreased with sunny weather (all three years), ♀♀ mating decreased (2003, 2004) and nectaring increased (♂♂: 2003 and 2004, ♀♀: 2004) with flight period duration, and ♂♂ perching followed domed patterns, indicating peaks in the middle of the adult period (all 3 years).
Regarding the within-habitat structures, the results were consistent across the three years for ♂♂ perching and patrolling. Perching prevailed near edges in all three years, plus outside of host plant patches in 2003. Patrolling consistently prevailed over centres of grasslands, i.e., further from woody edges. Patterns of ♂♂ chasing were inconsistent, prevailing at edges and in the centres depending on the year. Results for ♀♀ were also inconsistent, probably due to considerably lower sample sizes, but suggested that basking prevailed in the centres (2002, 2004) and near host plant (2003), while basking and nectaring prevailed outside the host plant patches (both 2003).
Discussion
Using two alternative approaches, timed observation of activities along a fixed transect and analysis of capture circumstances during mark-recapture, we linked within-habitat vegetation structures to adult activity patterns of the Euphydryas aurinia butterfly. Out of two mate-locating behaviours of ♂♂, perching was closely associated with meadow edges, i.e., with trees, woodland margins, lines of shrubs, or just unmanaged tall herbaceous vegetation surrounding regularly mown grasslands containing the host plants, Succisa pratensis. Patrolling, on the other hand, tended to occur at open short- to mid-sward grassland patches. Females concentrated at transect sections with high concentrations of host plants and nectar, which in turn caused a spatial separation of sexes. For conservation management of E. aurinia sites, it follows that in addition to efforts to maintain high host plant concentrations, the sites should contain some shrubs, edges, taller-sward and similar structures. The latter advice is sometimes mentioned as “heterogenous management” (e.g. van Swaay et al. 2012), but to our knowledge, until recently it lacked a quantitative support for E. aurinia.
Both our approaches produced complementary results and both withstood robust controls for short-term weather conditions and temporal aspects of adult flight. While the behavioural patterns due to weather (resting in windy overcast conditions, basking when the sward was wet by morning dew or after rains, behaviours associated with movements in sunny conditions) were rather trivial, the diurnal and the seasonal patterns displayed clear structuring. Interestingly, morning and late afternoon hours shared many similarities. The prevailing activities in 8:00–10:00 in the morning and 16:00–17:00 in the afternoon were basking, nectaring, and resting. This was arguably connected to the intake of energy, both thermal (cf. Franzen et al. 2022) and nutritional (cf. Botham et al. 2011) for commencing daily activities in the mornings, and to replenish the energy before night rest in the evenings (cf. Vlasanek et al. 2018). The energy demanding activities such as male perching (with frequent conspecific and heterospecific chases) and patrolling, and female oviposition, culminated in 11:00–14:00, i.e., around noon. Franzen et al. (2022) described peaks of E. aurinia activity in early afternoons, attributing it to the thermal requirements of this early-season species, and showed that activity tends to decline under extremely hot temperatures.
Whereas diel activity patterns displayed a similarity between mornings and evenings, seasonal patterns were linear, reflecting the changing proportion of sexes and changing status of the individuals along the flight period. Perching and patrolling peaked early in season, when ♂♂ prevailed in the studied colony (Zimmermann et al. 2011a) and were later followed by the peak of “maintenance activities”, especially nectaring. Vlasanek et al. (2018) observed this pattern for multiple temperate species with distinct generations. Notably, perching and patrolling were not segregated temporarily. Regarding ♀♀, besides later seasonal peaks of all activities attributable to protandry (Schtizckzelle et al. 2005; Zimmermann et al. 2011a), the ordination analysis showed that oviposition and nectaring were postponed relative to mating. A female must evidently have mated to oviposit, and intake of nectar presumably increases the number of egg batches produced (O’Brien et al. 2004).
The relationships between behaviours and vegetation structures remained apparent after considering the above weather and temporal effects. The most robust patterns concerned mate-locating activities. The association of perching with shrubs near short sward (ordination) and with edges (regressions) conforms with the classic (Scott 1974; Dennis and Shreeve 1998; Rutowski 1991; Wickman 1992) concept of perches as conspicuous landmarks in wind-shielded locations provided by edges of taller vegetation, but with good oversight of the habitat. Perches may occur at host plant patches (as in the skipper Carterocephalus palaemon (Pallas, 1771): Ravenscroft 1994, or the satyrine Coenonympha pamphilus (Linnaeus, 1758): Wickman 1985) or at patches of other critical resources, notably nectar (as in the copper Lycaena hippothoe (Linnaeus, 1761): Fischer and Fiedler 2001; Turlure and Van Dyck 2009). In our case, both the ordination and regressions suggested that the location of perches was independent of host plants distribution. Patrolling, on the other hand, occurs over mid-sward with a good supply of nectar, in the central parts of individual meadows (regressions) and rather close to host plants (ordination). Such a setting allows patrolling males to spot freshly hatched virgin females and at the same time, the unmated females to approach perching males. The latter consideration may deserve further attention, because Pinzari et al. (2019) observed that a fraction of females of Euphydryas aurinia provincials (Boisduval, 1828) mate a few days after emergence. The location of perches away from host plants indicates that perching males unlikely harass females seeking for oviposition, a situation described for L. hippothoe by Turlure and Van Dyck (2009), but does not exclude potential harassment of females by patrolling males.
The whole situation with perches situated near edges independently of host plants distribution, and males alternating perching with patrolling over host plants patches suggests that an ideal E. aurinia habitat would be finely structured, with shorter and taller swards, leeward edges, and host plant patches alternating at small scales of routine within-habitat movements. In such a setting, host plants with increased activity of females would naturally occur near edges with male perches. In traditional landscapes (cf. Loos et al. 2014; Perovic et al. 2015), and presumably in naturally patchy landscapes of prehistory (cf. Fahrig 2017), proximity of open short sward patches and edges of all types was probably a rule, and restoring highly heterogeneous conditions, e.g., by forest grazing (Saarinen et al. 2005), could boost E. aurinia populations. Several authors across the E. aurinia range disclosed “heterogeneity” as a positive correlate of its presence and density (Munguira et al. 1997; Scherer and Fartmann 2021), while others warned against uniformising management (Johansson et al. 2019). It is not unlikely that mate-seeking ♂♂, in contrast to ovipositing ♀♀, cannot recognise host plant patches, which may occasionally cause establishment of perches too far from females’ activity; such a situation was described in a patrolling butterfly, Parnassius mnemosyne (Linnaeus, 1758) (Konvička et al. 2007).
From a more general insect conservation perspective, detailed understanding of E. aurinia adult activity provides further evidence that large uniformly managed land units exceeding in size routine within-habitat movements (cf. Baguette and Van Dyck 2007), even if containing abundance of some resources (e.g., larval host plants), will always be inferior to patchily heterogeneous environment (cf. Liu et al. 2006). This knowledge, increasingly accepted by the conservation community over the past decades (Rundlöf and Smith 2006; Kivinen et al. 2008; Lebeau et al. 2015; Perovic et al. 2015; Schwarz and Fartmann 2022) gradually transferred into reserve management on small scales, including our study system. The practices applied to conserve grassland insects may include patchy mowing, retention of uncut fallows, or grazing via small panels. Regretfully, diversifying grasslands’ management remains difficult at large scales and beyond protected areas, including the matrix separating E. aurinia occupied sites in the western Czech Republic (Junker et al. 2021), although evidence is accumulating that much can be gained by relatively cheap measures, such as dissecting vast management units by temporary fallows or hedgerows (Buri et al. 2013; Bruppacher et al. 2016; Salek et al. 2018).
References
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185
Baguette M, Van Dyck H (2007) Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landsc Ecol 22:1117–1129
Bennett VJ, Betts MG, Smith WP (2014) Influence of thermal conditions on habitat use by a rare spring-emerging butterfly Euphydryas editha taylori. J Appl Entomol 138:623–634
Botham MS, Ash D, Aspey N, Bourn NAD, Bulman CR, Roy DB, Swain J, Zannese A, Pywell RF (2011) The effects of habitat fragmentation on niche requirements of the marsh fritillary, Euphydryas aurinia (Rottemburg, 1775) on calcareous grasslands in southern UK. J Insect Conserv 15:269–277
Bruppacher L, Pellet J, Arlettaz R, Humbert J-Y (2016) Simple modifications of mowing regime promote butterflies in extensively managed meadows: evidence from field-scale experiments. Biol Conserv 196:196–202
Bulman CR, Wilson RJ, Holt AR, Bravo LG, Early RI, Warren MS, Thomas CD (2007) Minimum viable metapopulation size, extinction debt, and the conservation of a declining species. Ecol Appl 17:1460–1473
Buri P, Arlettaz R, Humbert JY (2013) Delaying mowing and leaving uncut refuges boosts orthopterans in extensively managed meadows: evidence drawn from field-scale experimentation. Agr Ecosyst Environ 181:22–30
Caro T (2007) Behavior and conservation: a bridge too far? Trends Ecol Evol 22:394–400
Courtney SP, Duggan AE (1983) The population biology of the orange tip butterfly Anthocharis cardamines in Britain. Ecol Entomol 8:271–281
Davis ML, Barker C, Powell I, Porter K, Ashton P (2021) Combining modelling, field data and genetic variation to understand the post-reintroduction population genetics of the Marsh Fritillary butterfly (Euphydryas aurinia). J Insect Conserv 25:875–886
Dennis RLH (2010) A resource-based habitat view for conservation: butterflies in the British landscape. Wiley-Blackwell, p 420
Dennis RLH, Shreeve TG (1998) Hostplant-habitat structure and the evolution of butterfly mate-locating behaviour. Zool J Linn Soc 94:301–318
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Ann Rev Ecol Evol Syst 48:1–23
Fischer K, Fiedler K (2001) Resource-based territoriality in the butterfly Lycaena hippothoe and environmentally induced behavioural shifts. Anim Behav 61:723–732
Franzén M, Francioli Y, Askling J, Kindvall O, Johansson V, Forsman A (2022) Differences in phenology, daily timing of activity, and associations of temperature utilization with survival in three threatened butterflies. Sci Rep 12:7534
Fric Z, Hula V, Klimova M, Zimmermann K, Konvicka M (2010) Dispersal of four fritillary butterflies within identical landscape. Ecol Res 25:543–552
Ghidotti S, Cerrato C, Casacci LP, Barbero F, Paveto M, Pesce M, Plazio E, Rocchia E, Panizza G, Balletto E, Viterbi R, Bani L, Bonelli S (2018) Scale-dependent resource use in the Euphydryas aurinia complex. J Insect Conserv 22:593–605
Hula V, Konvicka M, Pavlicko A, Fric Z (2004) Marsh Fritillary (Euphydryas aurinia) in the Czech Republic: monitoring, metapopulation structure, and conservation of an endangered butterfly. Entomol Fennica 15:231–241
Janz N (2005) The relationship between habitat selection and preference for adult and larval food resources in the polyphagous butterfly Vanessa cardui (Lepidoptera: Nymphalidae). J Insect Behav 18:767–780
Johansson V, Kindvall O, Askling J, Franzen M (2019) Intense grazing of calcareous grasslands has negative consequences for the threatened marsh fritillary butterfly. Biol Conserv 239:108280
Johansson V, Kindvall O, Askling J, Franzén M (2020) Extreme weather affects colonization-extinction dynamics and the persistence of a threatened butterfly. J Appl Ecol 57:1068–1077
Junker M, Schmitt T (2010) Demography, dispersal and movement pattern of Euphydryas aurinia (Lepidoptera: Nymphalidae) at the Iberian Peninsula: an alarming example in an increasingly fragmented landscape? J Insect Conserv 14:237–246
Junker M, Wagner S, Gros P, Schmitt T (2010) Changing demography and dispersal behaviour: ecological adaptations in an alpine butterfly. Oecologia 164:971–980
Junker M, Zimmermann M, Ramos A, Gros P, Konvicka M, Nève G, Rakosy L, Tammaru T, Castilho R, Schmitt T (2015) Three in one-multiple faunal elements within an endangered European butterfly species. PLoS ONE 10:e0142282
Junker M, Konvicka M, Zimmermann K, Schmitt T (2021) Gene-flow within a butterfly metapopulation: the marsh fritillary Euphydryas aurinia in western Bohemia (Czech Republic). J Insect Conserv 25:585–596
Kivinen S, Luoto M, Heikkinen RK, Saarinen K, Ryttari T (2008) Threat spots and environmental determinants of red-listed plant, butterfly and bird species in boreal agricultural environments. Biodivers Conserv 17:3289–3305
Konvicka M, Vlasanek P, Hauck D (2007) Absence of forest mantles creates ecological traps for Parnassius mnemosyne (Papilionidae). Nota Lepid 29:145–152
Korb S, Bolshakov LV, Fric ZF, Bartonova A (2016) Cluster biodiversity as a multidimensional structure evolution strategy: Checkerspot butterflies of the group Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera: Nymphalidae). Syst Entomol 41:441–457
Lebeau J, Wesselingh RA, Van Dyck H (2015) Butterfly density and behaviour in uncut hay meadow strips: behavioural ecological consequences of an agri-environmental scheme. PLoS ONE 10:e0134945
Liu W, Wang Y, Xu R (2006) Habitat utilization by ovipositing females and larvae of the Marsh fritillary (Euphydryas aurinia) in a mosaic of meadows and croplands. J Insect Conserv 10:351–360
Loos J, Dorresteijn I, Hanspach J, Fust P, Rakosy L, Fischer J (2014) Low-intensity agricultural landscapes in transylvania support high butterfly diversity: implications for conservation. PLoS ONE 9:e103256
Maes D, Jacobs I, Segers N, Vanreusel W, Van Daele T, Laurijssens G, Van Dyck H (2014) A resource-based conservation approach for an endangered ecotone species: the Ilex Hairstreak (Satyrium ilicis) in Flanders (north Belgium). J Insect Conserv 18:939–950
McKay HV (1991) Egg-laying requirements of woodland butterflies—brimstones (Gonepteryx rhamni) and alder buckthorn (Frangula alnus). J Appl Ecol 28:731–743
Meister H, Lindman L, Tammaru T (2015) Testing for local monophagy in the regionally oligophagous Euphydryas aurinia (Lepidoptera: Nymphalidae). J Insect Conserv 19:691–702
Munguira ML, Martin J, GarciaBarros E, Viejo JL (1997) Use of space and resources in a Mediterranean population of the butterfly Euphydryas aurinia. Acta Oecologica 18:597–612
Murphy DD, Menninger MS, Ehrlich PR (1984) Nectar source distribution as a determinant of oviposition host species in Euphydryas chalcedona. Oecologia 62:269–271
O’Brien DM, Boggs CL, Fogel ML (2004) Making eggs from nectar: the role of life history and dietary carbon turnover in butterfly reproductive resource allocation. Oikos 105:279–291
Pennekamp F, Garcia-Pereira P, Schmitt T (2014) Habitat requirements and dispersal ability of the Spanish Fritillary (Euphydryas desfontainii) in southern Portugal: evidence-based conservation suggestions for an endangered taxon. J Insect Conserv 18:497–508
Perovic D, Gámez-Virués S, Börschig C, Klein AM, Krauss J, Steckel J, Rothenwöhrer C, Erasmi S, Tscharntke T, Westphal C (2015) Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J Appl Ecol 52:505–513
Pertoldi C, Ruiz-Gonzalez A, Bahrndorff S, Lauridsen NR, Henriksen TN, Eskildsen A, Hoye TT (2021) Strong isolation by distance among local populations of an endangered butterfly species (Euphydryas aurinia). Ecol Evol 11:12790–12800
Pielech R, Zajac K, Kadej M, Malicki M, Malkiewicz A, Tarnawski D (2017) Ellenberg’s indicator values support prediction of suitable habitat for pre-diapause larvae of endangered butterfly Euphydryas aurinia. PLoS ONE 12:e0179026
Pinzari M, Pinzari M, Sbordoni V (2019) Make it simple: mating behaviour of Euphydryas aurinia provincialis (Lepidoptera: Nymphalidae). Eur Zool J 86:220–232
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation. Chapman and Hall, London, p 244
Pschera J, Warren JM (2018) Microhabitat selection by ovipositing females and pre-diapause larvae of a Welsh population of Euphydryas aurinia (Lepidoptera: Nymphalidae). J Insect Conserv 22:571–579
Ravenscroft NOM (1994) Environmental influences on mate location in male chequered skipper butterflies, Carterocephalus palaemon (Lepidoptera: Hesperiidae). Anim Behav 47:1179–1187
Rundlöf M, Smith HG (2006) The effect of organic farming on butterfly diversity depends on landscape context. J Appl Ecol 43:1121–1127
Rutowski RL (1991) The evolution of male mate-location behavior in butterflies. Am Nat 138:1121–1139
Saarinen K, Juntunen J, Valtonen A (2005) Resumed forest grazing restored a population of Euphydryas aurinia (Lepidoptera: Nymphalidae) in SE Finland. Eur J Entomol 102:683–690
Salek M, Hula V, Kipson M, Dankova R, Niedobova J (2018) A Bringing diversity back to agriculture: smaller fields and non-crop elements enhance biodiversity in intensively managed arable farmlands. Ecol Indic 90:65–73
Samways MJ (2007) Insect conservation: a synthetic management approach. Ann Rev Entomol 52:465–487
Scherer G, Fartmann T (2021) Occurrence of an endangered grassland butterfly is mainly driven by habitat heterogeneity, food availability, and microclimate. Insect Sc, Early View,. https://doi.org/10.1111/1744-7917.12975
Schtickzelle N, Choutt J, Goffart P, Fichefet V, Baguette M (2005) Metapopulation dynamics and conservation of the marsh fritillary butterfly: population viability analysis and management options for a critically endangered species in Western Europe. Biol Conserv 126:569–581
Schwarz C, Fartmann T (2022) Traditional grazing management creates heterogeneous swards and fosters grasshopper densities. Insect Sci. https://doi.org/10.1111/1744-7917.13041
Scott JA (1974) Mate-locating behavior of butterflies. Am Midl Nat 91:103–117
Settele J, Dover J, Konvicka M, Dolek M (2009) Butterflies of European ecosystems: impact of land use and options for conservation management. In: Settele J, Shreeve T, Konvicka M, van Dyck H (eds) The ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 353–370
Shreeve TG, Dennis RLH (2011) Landscape scale conservation: resources, behaviour, the matrix and opportunities. J Insect Conserv 15:179–188
Sielezniew M, Kostro-Ambroziak A, Klimczuk P, Deoniziak K, Palka K, Nowicki P (2019) Habitat-related differences in the adult longevity of two ecotypes of a specialized butterfly. J Zool 307:93–103
Sigaard P, Pertoldi C, Madsen AB, Sogaard B, Loeschcke V (2008) Patterns of genetic variation in isolated Danish populations of the endangered butterfly Euphydryas aurinia. Biol J Linn Soc 95:677–687
Singer MC, Stefanescu C, Pen I (2002) When random sampling does not work: standard design falsely indicates maladaptive host preferences in a butterfly. Ecol Lett 5:1–6
Swartz MT, Ferster B, Vulinec K, Paulson G (2015) Measuring regal fritillary butterfly (Speyeria idalia) habitat requirements in south-central Pennsylvania: implications for the conservation of an imperiled butterfly. Northeast Nat 22:812–829
Ter Braak CJF, Smilauer P (2012) Canoco reference manual and user’s guide: software for ordination, version 5.0. Microcomputer Power, Ithaca, p 496
Tjørnløv RS, Kissling WD, Barnagaud JY, Bocher PK, Hoye TT (2015) Oviposition site selection of an endangered butterfly at local spatial scales. J Insect Conserv 19:377–391
Tolman T, Lewington R (2009) Collins butterfly guide: the most complete guide to the butterflies of Britain and Europe. HarperCollins, London, pp 384
Turlure C, Van Dyck H (2009) On the consequences of aggressive male mate-locating behaviour and micro-climate for female host plant use in the butterfly Lycaena hippothoe. Behav Ecol Sociobiol 63:1581–1591
Turlure C, Radchuk V, Baguette M, Van Dyck H, Schtickzelle N (2011) On the significance of structural vegetation elements for caterpillar thermoregulation in two peat bog butterflies: Boloria eunomia and B. aquilonaris. J Therm Biol 36:173–180
Turlure C, Schtickzelle N, Dubois Q, Baguette M, Dennis RLH, Van Dyck H (2019) Suitability and transferability of the resource-based habitat concept: a test with an assemblage of butterflies. Front Ecol Evol 7:127
van Swaay C, Collins S, Dusej G, Maes D, Munguira ML, Rakosy L, Ryrholm N, Šašić M, Settele J, Thomas JA, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhoff I (2012) Dos and don’ts for butterflies of the habitats directive of the European union. Nature Conserv 1:73–153
Vanreusel W, Van Dyck H (2007) When functional habitat does not match vegetation types: a resource-based approach to map butterfly habitat. Biol Conserv 135:202–211
Vlasanek P, Fric ZF, Zimmermann K, Novotny D, Cizek O, Kleckova I, Vrba P, Kadlec T, Konvicka M (2018) Do butterfly activity data from mark-recapture surveys reflect temporal patterns? J Insect Behav 31:385–401
Vrba P, Grill S, Kadlec T, Papaj V, Konvicka M (2021) How do adults of the critically endangered hermit butterfly (Chazara briseis) utilise their habitat? (Lepidoptera, Satyrinae). J Insect Conserv 25:39–48
Wahlberg N (2000) Comparative descriptions of the immature stages and ecology of five Finnish melitaeine butterfly species (Lepidoptera: Nymphalidae). Entomol Fennica 11:167–174
Wahlberg N, Kullberg J, Hanski I (2001) Natural history of some Siberian melitaeine butterfly species (Nymphalidae : Melitaeini) and their parasitoids. Entomol Fennica 12:72–77
Wahlberg N, Klemetti T, Hanski I (2002) Dynamic populations in a dynamic landscape: the metapopulation structure of the marsh fritillary butterfly. Ecography 25:224–232
Wang RJ, Wang YF, Lei GC, Xu RM, Painter J (2003) Genetic differentiation within metapopulations of Euphydryas aurinia and Melitaea phoebe in China. Biochem Genet 41:107–118
Warren MS (1994) The UK status and suspected metapopulation structure of a threatened European butterfly, the Marsh Fritillary Eurodryas aurinia. Biol Conserv 67:239–249
Warren MS, Maes D, van Swaay CAM, Goffart P, Van Dyck H, Bourn NAD, Wynhoff I, Hoare D, Ellis S (2021) The decline of butterflies in Europe: problems, significance, and possible solutions. Proc Nat Acad Sc USA 118:e2002551117
Wickman PO (1985) Territorial defence and mating success in males of the small heath butterfly, Coenonympha pamphilus L. (Lepidoptera: Satyridae). Anim Behav 33:1162–1168
Wickman PO (1992) Sexual selection and butterfly design—a comparative study. Evolution 46:1525–1536
Zimmermann K, Blazkova P, Cizek O, Fric Z, Hula V, Kepka P, Novotny D, Slamova I, Konvicka M (2011a) Adult demography in the Marsh fritillary butterfly, Euphydryas aurinia (Rottenburg, 1775) in the Czech Republic: patterns across sites and seasons. Eur J Entomol 108:243–254
Zimmermann K, Fric Z, Jiskra P, Kopeckova M, Vlasanek P, Zapletal M, Konvicka M (2011b) Mark-recapture on large spatial scale reveals long distance dispersal in the Marsh Fritillary, Euphydryas aurinia. Ecol Entomol 36:499–510
Acknowledgements
We are grateful to the Czech Science Foundation (P505/10/2167), South Bohemian University (144/2010/100), and the Technology Agency of the Czech Republic (SS01010526) for financial support. Matthew Sweney corrected the English.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
All authors conceptualised and designed the study and all of them equally participated in collecting the data in the field. KZ and MK curated the data in time between the fieldwork and manuscript preparation. MK, PV and ZFF performed the analyses; ZFF prepared the artworks, MK and ZFF wrote the article. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10841_2023_459_MOESM1_ESM.xlsx
Supplementary file1 (XLSX 154 kb)—Appendix 1. Spreadsheet containing primary data that entered the ordination analyses of adult Euphydryas aurinia behavioural records along timed fixed transect.
10841_2023_459_MOESM2_ESM.xlsx
Supplementary file2 (XLSX 301 kb)—Appendix 2. Spreadsheet (three separate leaves for years 2002 – 2004) containing primary data used in binomial regression analyses of behavioural records obtained during mark-recapture studies adult Euphydryas aurinia butterfly.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Konvicka, M., Hula, V., Vlasanek, P. et al. Within-habitat vegetation structure and adult activity patterns of the declining butterfly Euphydryas aurinia. J Insect Conserv 27, 335–346 (2023). https://doi.org/10.1007/s10841-023-00459-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00459-x