Abstract
The mountain ecosystems of Central Europe are an important natural phenomenon. The character of small isolated islands also predetermines their vulnerability. Typical inhabitant of subalpine and alpine peat bogs, tiny montane habitats, is the endangered dragonfly Alpine Emerald (Somatochlora alpestris) a glacial relict surviving in restricted area of several mountain ranges within Central Europe. Species is threatened mainly by habitat loss and its transformation due to climate change, the expansion of tourist activities and plant succession. In our study from three mountain ranges in the Czech Republic, we bring the first ever evidence of successful development of S. alpestris in artificial habitats. Successful development of the species was recorded in peat pools created by the movement of heavy machinery on now almost abandoned forest roads. Some of the pools have been colonized in great numbers - up to tens of larvae of different instars, exuviae and imagoes have been found. Successful colonisation of the species was mainly due to: (i) proximity to source sites, (ii) suitable environmental parameters of the secondary habitat and (iii) the gradual abandonment of the paths´ use, leading to a reduction in the frequency of disturbance.
Implications for insect conservation
We consider active management through the pools´ creation as a possible way to support S. alpestris populations, both by (i) establishing new sites replacing lost ones, but also by (ii) creating stepping stones allowing more effective dispersal of individuals in the environment and functionally connecting natural habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ecosystems of the Central European mountains represent some of the most important natural environments in Europe. The subalpine and alpine habitats in particular stand out for their high natural value, representing isolated islands with uniquely formed communities with many endemic animals and plants (Nagy et al. 2003). The relict dragonfly Alpine Emerald (Somatochlora alpestris) (= Salp) is one of the typical species associated with these rare and small mountain habitats. Salp is Eurosiberian species with a boreo-montane distribution. In Central Europe it occurs disjunctively only in the highest mountain ranges – the Vosges, the whole Alpine range, the Schwarzwald, Harz, Thüringer Wald, most of the Sudeten Mountains (Šumava, Slavkovský les, Jizerské hory, Orlické hory, Králický Sněžník and Hrubý Jeseník), similarly in several places in the Carpathians (High Tatras, Orava Beskydy, Ukrainian Carpathians and Southern Carpathians in Romania) (Dijkstra and Lewington 2006; Dolný et al. 2007; Wildermuth 2008; Flenker 2011).
One of the most numerous populations in the Czech Republic in the Hrubý Jeseník mountains is bound to pools, which together cover an area equivalent to a small garden, i.e. hundreds of m2 (Holuša 1995, 1997). Therefore, this population is also potentially threatened with extinction (Griffen and Drake 2008). Hence the conservation of this species is problematic and relies on the strict protection of the natural habitat, i.e. mostly on the preservation of its hydrological regime and leaving the surface of the peatland untouched. However, subalpine habitats are changing and becoming overgrown due to climate change, spread of woody plants, loss of grazing and tourism development (Kašák et al. 2013, 2015; Zeidler et al. 2020; Šenfeldr et al. 2021), threatening populations of relict mountain insect species (Kašák et al. 2015; Konvicka et al. 2021). Therefore, alternative methods for dragonflies´ conservation are being sought in the form of active management - including the support of populations through the creation of artificial habitats. However, studies on the establishment and successful use of artificial aquatic habitats for dragonflies in mountains are still very rare (but see Dolný et al. 2018; Fait et al. 2020).
Salp is a dragonfly with specific ecological requirements, it is a typical species for upland areas on flat ridges and especially in mountain saddles, where the larvae develop in small pools or peat ponds. It prefers stagnant waters for its development, but occasionally occurs in spring biotopes. The species has a strong preference for open montane to alpine sites, with occasional occurrences in lower elevations in open forests. Most of the localities with documented development in the Czech Republic are above 1000 m (Dolný et al. 2007), but in the Alps sites up to about 2500 m are inhabited (Janetschek 1960; Wildermuth 2008). The water area of the preferred habitats is mostly limited to a few m2. The species is associated with water bodies with a depth of up to 30 cm and a bottom consisting of a muddy layer of at least 10 cm and a low plant cover of up to 20% of the surface area. The banks are mostly sharply delimited (often perpendicular to undercut) and the littoral of the sites, depending on their character and altitude, is characterised by vegetation with a significant representation of peat mosses (Sphagnum spp.), cottongrasses (Eriophorum spp.), cranberry (Oxycoccus palustris), sedges (e.g. Carex limosa) and polytrichum mosses (Polytrichum spp.). At the lower elevation sites, only Norway spruce (Picea abies) and dwarf pine (Pinus mugo) are present among the woody plants in the surrounding area (Holuša 1995, 1997; Wildermuth 1999, 2008; Dijkstra and Lewington 2006; Dolný et al. 2007; Oertli 2010; De Knijf et al. 2011).
The biotopes are also characterised by a frequently fluctuating water regime. Salp larvae living on the bottom and in muddy sediments are able to survive extreme conditions such as summer drying of the habitat for up to 3 months, then even subsequent winter freezing. Larval development is long, and it has been shown to take more than 3 years (Johansson and Nilsson 1991). With a few exceptions, Salp larvae have so far only been found in natural habitats (Wildermuth 1999; Dolný et al. 2007; Fait et al. 2020). Adults are active from late June to early August. Imagoes are characterized by relatively low fidelity to the home range, as well as good dispersal ability (Wildermuth 1999; Knaus and Wildermuth 2002; Dolný et al. 2007).
The specific and extreme conditions of the Salp habitats (Wildermuth and Knaus 2002) predetermine the very poor insect community of these sites.The Alpine Emerald is thus usually the only dragonfly species permanently inhabiting these aquatic habitats. The only typical species accompanying Salp on its habitats in the Czech Republic is the Common Hawker (Aeshna juncea) (Holuša 1995, 1997), on larger peat ponds the Bog Hawker (A. subarctica), Common Bluet (Enallagma cyathigerum), Small Whiteface (Leucorrhinia dubia) and individually other tyrphophilic species, and on alpine peat bogs the Azure Hawker (Aeshna caerulea) (Dolný et al. 2007).
Salp is listed on the Red Lists of many countries (e.g. Raab et al. 2006; Dolný et al. 2017; Monnerat et al. 2021; Ott et al. 2021). Therefore, the present paper is the first to: (i) report on the successful development of Salp in artificial habitats and (ii) propose options for artificial habitat creation as a tool to support the population of a relict dragonfly species – Salp.
Methods
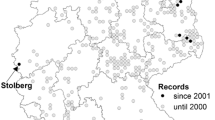
The study from the Czech Republic analyses data collected in three Central European mountain ranges of the High Sudetes − Hrubý Jeseník Mts., Králický Sněžník Mts. and Rychlebské hory Mts., (Fig. 1). The altitudes of the highest ridges of these mountains range from 1000 to 1490 m above sea level and, except for the Rychlebské hory Mts., a subalpine forest-free zone has developed. The climate is cold, with mean temperatures varying from − 6.6° C in the coldest month (January) to 9.5° C in the warmest month (August). The long-term annual average rainfall is 1200–1300 mm and 200 rainy days per year on average. Snow cover lasts about 180 days, typically from the end of October to mid-May (Demek and Kříž 1994). Salp is known of all three mountain ranges (Dolný et al. 2007), in the case of some localities for a long time since the 19th century (Kolenati 1859). Here, Salp is associated with saddle peatlands on ridges ranging from loose spruce forest to subalpine zone, with only a few occupied water patches in each mountain range (Dolný et al. 2007).
The research was carried out at 4 sites: Hrubý Jeseník – Ostrý vrch Mt. (= OV) GPS: 50.0818 N, 17.2604E, Velká Jezerná Mt. (= VJ) GPS: 50.0684 N, 17.1836E; Králický Sněžník – Tetřeví hora Mt. (= TH) GPS: 50.1678 N, 16.8650E (Fig. 2) and Rychlebské hory – Smrk Mt. (= SM) GPS: 50.2302 N, 17.0304E. A total of 12 artificial pools were surveyed (OV = 2, VJ = 3, TH = 5, SM = 2). In the vicinity of each site (0.1–2.3 km) there are natural habitats inhabited by Salp (Holuša 1997; Dolný et al. 2007; Dolný 2009). The anthropogenically created aquatic habitats studied were always formed by pools in forest (unpaved) roads in the rutted tracks left by heavy forestry equipment. Mostly these were old, abandoned for more than 10 years, or rarely used (estimated once every few years) forest roads. The aquatic habitats were created usually during timber export after wind disturbance and following bark beetle outbreak (recorded on the basis of aerial photographs), which gave rise to semi- to fully open habitats surrounded by loose young spruce stands up to 30 years old (Kašák et al. 2017).
Artificially created pool in an abandoned forest path inhabited by Alpine Emerald (Somatochlora alpestris). The image shows the pool with the highest abundance of the species, where 30 larvae of different instars, several exuviae and a patrolling imagoes were recorded. The creation of artificial habitats of this type can therefore serve as a tool for supporting populations of this endangered dragonfly (Králický Sněžník - Tetřeví hora Mt., Czech Republic, 13 July 2021, photo J. Kašák)
Data sampling
For the purpose of extensive dragonfly monitoring (2016–2022, two visits of each site), each site was visited during one vegetation season (June – August). The characteristics of each artificial aquatic habitat were recorded: minimum distance to natural pool occupied by Salp (km), altitude (m a.s.l.), water surface area (m2), maximum depth (cm), chemical parameters (pH and conductivity), composition of riparian and aquatic vegetation, presence of deep muddy bottom (present/absent), disturbance (present = visible disturbance of vegetation, reduced cover; absent = minimal or no disturbance of vegetation, high cover of vegetation). The chemical parameters were measured once with an Extech EC500 instrument. The nearshore areas including aquatic vegetation and bottom were hand surveyed and dragonflies larvae were collected. The banks were surveyed once for 10 min on each site. Exuviae and adults were recorded simultaneously. Determination of larvae and exuviae was made according to Heidemann and Seidenbusch (2002) and Brochard et al. (2012).
Results
All stages of development of Salp have been found, including larvae of different instars (ranging from youngest - size ca. 1 cm - to older just before metamorphosis) exuviae and adults, demonstrating the development of multiple generations. Larvae were found in all four study sites (i.e. mountains – OV, VJ, TH and SM), with evidence of species development in 6 of the 12 artificial pools. Nearest inhabited natural peat-bog from each study site was located within 0.1–2.3 km range. The species was abundant in some pools, as evidenced by the finding of up to 30 larvae (Table 1). These records represent the first evidence of repeated successful use of artificial aquatic habitats by Salp. The inhabited pools were small with a maximum area of 20 m2 and depth 70 cm. Typical was a bottom with deep muddy layer (even tens of cm) and richly developed riparian vegetation, where peat and polytrichum moss, together with sedges were significantly represented. Pools with Salp were typically colonised by other species such as A. juncea, A. cyanea and P. nymphula (Table 1). In contrast, unoccupied pools were usually dominated by coarser substrate in the bottom (gravel, stones or a layer of fallen wood) and were still disturbed (car runs and mud wading animals). The banks were sparsely vegetated (sometimes almost barren), usually dominated by Juncus sp. and Avenella flexuosa, and were not colonised by other dragonfly species, except from A. cyanea in one pool (although Salp males were sometimes observed patrolling here). Another tyrphobiont species as A. subarctica and L. dubia were not recorded at artificial pools (Table 1).
The characteristics (elevation, area, depth, bottom substrate and vegetation) of the artificial habitats colonised by Salp correspond to natural peatlands (Fig. 2). Similar conditions are also illustrated by the partial overlap of “syntopic” species such as the typical species accompanying Salp on its sites, above all, the tyrphophilous A. juncea.
Discussion
Salp is an endangered glacial relict associated with very specific natural habitats – alpine peat bogs (Dolný et al. 2007). According to previous studies, the creation of new habitats for such highly specialized tyrphobionts has not yet ensured the conditions for successful development and long-term persistence of populations (Dolný et al. 2018; Fait et al. 2020). Apparently, these small-sized artificial pools are not attractive habitat for other specialized dragonflies – for instance, A. subartica and L. dubia prefer larger peat-bogs with abundant floating vegetation of peat moss (Dolný et al. 2007). The present study, however, demonstrates the successful development of Salp larvae in artificial anthropogenic habitats created by the passage of forestry machinery (Fig. 2; Table 1).
Our surprising findings can be explained by a combination of factors: (1) the location and geomorphology of the sites, and (2) the origin and subsequent use of the biotopes. All study sites with artificial habitats are located in the mountain ranges where the Salp population is found (Dolný et al. 2007) and the nearest inhabited peat-bogs were no more than 2.3 km away (Holuša 1997; Dolný 2009). The colonisation attempts of Salp imagoes thus may have been frequent due to the proximity of occupied peatlands. Flights of Salp imagoes between sites within 1 km are common, within 2 km rare and the longest known to date is 7 km (Knaus and Wildermuth 2002). At the same time, the artificial biotopes are located in climatically identical places and also have a similar hydrological regime due to the geomorphology (Table 1). The newly created habitats are on the flat ridges of mountains and saddles, where there are naturally moist, gentle terrain depressions with areas of peatland vegetation, mainly covered with peat moss. The favourable geomorphology and hydrological regime thus allows the creation of permanent or at least longer-lasting aquatic habitats after disturbance.
The second factor contributing to the successful persistence of Salp populations is the mean of habitat creation and subsequent development. Artificial habitats are represented by pools in old forest roads, mostly created in rutted tracks on roads that had been short and intensively used for a few years after spruce logging and then were abandoned. The movement of heavy equipment in the soft waterlogged substrate led to the formation of depressions, several tens of cm deep, that were flooded with water. Natural succession has subsequently led to the creation of habitats that are very close in character to natural peat-bogs with typically developed vegetation (Results, Fig. 2).
We hypothesize that other attempts to create artificial habitats (Dolný et al. 2018; Fait et al. 2020) for support of Salp populations have not been successful mainly due to the following habitat characteristics: lower elevation (compared to the species’ optimum), lack of a source population nearby (see Dolný et al. 2018), larger water surface area, greater depth and limited vegetation development (see Fait et al. 2020).
Conservation summary
In conclusion, the observed development of Salp in artificial habitats can be seen as a surprising opportunity for the conservation of this species, especially in case of disappearance of some of the existing sites. Artificial habitats for dragonflies in the mountains may be important as changes in the water regime (e.g. drying) occur due to warming. At least, these habitats may act as stepping stones as suggested by a recent study from the Alps (Fait et al. 2020).
Nevertheless, a significant factor affecting dragonfly survival in artificial small-scale habitats is the appropriate level and frequency of disturbance. On the one hand, succession threatens to overgrow pools with taller vegetation and woody plants, shading and subsequent grounding of habitats. But on the other hand, due to the small area, it is easy to destroy these habitats by a medium-strong disturbance (cf. Harabiš and Dolný 2015).
Based on the presented results and knowledge of the species’ bionomy, we propose to: (i) create new habitats within a maximum of a few km (optimally 1–2 km) from existing occupied areas and in places with similar geomorphology - i.e. flat saddles with wetland tyrphophilous vegetation; (ii) create more pools (each of several m2) in the locality; (iii) maintain an appropriate level of disturbance, e.g., reduce the use of heavy machinery for the part of an area, with the reciprocal change within a few years (preferably outside the growing season), mechanically expose part of the pool banks (e.g. with a spade), remove the part of the muddy layer (all the found larvae return back to the pool), (iv) keep the immediate surroundings of the pools open (removing woody plants); (v) as this is the first report on the support of the Salp population by the creation of new habitats, the long-term development of the populations as well as its habitats is worth-monitoring, for an effective application of this management in the conservation of the species.
References
Brochard C, Groenendijk D, van der Ploeg E, Termaat T (2012) Fotogids Larvenhuidjes van Libellen. KNNV Uitgeverij, Zeist
De Knijf G, Flenkerb U, Vanappelghemc C, Mancid CO, Kalkmane VJ, Demolder H (2011) The status of two boreo-alpine species, Somatochlora alpestris and S. arctica, in Romania and their vulnerability to the impact of climate change (Odonata: Corduliidae). I J Odonatol 14:111–126
Dijkstra K-D, Lewington R (2006) Field Guide to the Dragonflies of Britain Europe including western Turkey and north-western Africa. British Wildlife Publishing, Gillingham, Dorset
Dolný A (2009) Aktualizovaná analýza vážek v Národních přírodních rezervacích CHKO Jeseníky. IX Svatováclavské česko-polsko-německé setkání Jeseník 2009:19–24
Dolný A, Harabiš F, Holuša O, Hanel L, Waldhauser M (2017) Odonata (Vážky). pp. 118–122 In: Hejda R, Farkač J & Chobot K (eds) Červený seznam ohrožených druhů České republiky. Bezobratlí. Příroda, Praha, 36:1–612
Dolný A, Šigutová H, Ožana S, Choleva L (2018) How difficult is it to reintroduce a dragonfly? Fifteen years monitoring Leucorrhinia dubia at the receiving site. Biol Conserv 218:110–117. https://doi.org/10.1016/j.biocon.2017.12.011
Fait P, Demierre E, Ilg C, Oertli B (2020) Small mountain reservoirs in the Alps: new habitats for alpinefreshwater biodiversity? Aquat Conserv: Mar Freshw Ecosyst 30:617–630. https://doi.org/10.1002/aqc.3306
Flenker U (2011) Odonata of the romanian Carpathians with notes on Somatochlora alpestris and on the first romanian record of Aeshna subarctica (Odonata: Corduliidae, Aeshnidae). Libellula 30(3/4):183–202
Griffen BD, Drake JM (2008) Effects of habitat quality and size on extinction in experimental populations. Proc R Soc B 275:2251–2256. https://doi.org/10.1098/rspb.2008.0518
Harabiš F, Dolný A (2015) Odonates need natural disturbances: how human-induced dynamics affect the diversity of dragonfly assemblages. Freshw Sci 34:1050–1057. https://doi.org/10.1086/682234
Heidemann H, Seidenbusch R (2002) Die Libellenlarven Deutschlands. Die Tierwelt Deutschlands 72
Holuša O (1995) Výskyt vážek rodu Somatochlora na území bývalého Československa (Odonata: Corduliidae). Klapalekiana 31:101–110
Holuša O (1997) Nové znalosti o rozšíření vážek rodu Somatochlora na území bývalého Československa (Odonata: Corduliidae). Klapalekiana 33:23–28
Dolný A, Bárta D, Holuša O, Waldhauser M, Hanel L (2007) The Dragonflies of the Czech Republic: Ecology, conservation and distribution. Cesky svaz ochrancu prırody Vlasim, Vlasim, pp 472–477
Janetschek H (1960) Die Alpen von Zell am See bis Bregenz. Exkursionsführer XI. Int. Entomologenkongress, Wien 1960:115–19
Johansson F, Nilson AN (1991) Freezing tolerance and drought resistence of Somatochlora alpestris (Seylys) larvae in boreal temporary pools (Anisoptera: Corduliidae). Odonatologica 20:245–252
Kašák J, Mazalová M, Šipoš J, Kuras T (2013) The effect of alpine ski-slopes on epigeic beetles: does even a nature-friendly management make a change? J Insect Conserv 17:975–988. https://doi.org/10.1007/s10841-013-9579-3
Kašák J, Mazalová M, Šipoš J, Kuras T (2015) Dwarf pine - invasive plant threatens biodiversity of alpine beetles. Biodivers Conserv 24:2399–2415. https://doi.org/10.1007/s10531-015-0929-1
Kašák J, Foit J, Hučín M (2017) Succession of ground beetles (Coleoptera: Carabidae) communities after windthrow disturbance in a montane Norway spruce forest in the Hrubý Jeseník Mts. (Czech Republic. J For Sci 63:180–187
Knaus P, Wildermuth H (2002) Site attachment and displacement of adults in two alpine metapopulations of Somatochlora alpestris (Odonata: Corduliidae). I J Odonatol 5:111–128. https://doi.org/10.1080/13887890.2002.9748183
Kolenati F (1859) Fauna des Altvaters (Hohen Gesenkes der Sudeten). Buchdruckerei Rudolf Rohrer’s Erben, Brünn
Konvicka M, Kuras T, Liparova J, Slezak V, Horazna D, Klecka J, Kleckova (2021) I. Low winter precipitation, but not warm autumns and springs, threatens mountain butterflies in middle-high mountains. PeerJ 9:12021. https://doi.org/10.7717/peerj.12021
Monnerat C, Wildermuth H, Gonseth Y (2021) Rote Liste der Libellen. Gefährdete Arten der Schweiz. Umwelt-Vollzug Nr. 2120. Bundesamt für Umwelt
Nagy L, Grabherr G, Körner Ch, Thompson DBA (2003) Alpine biodiversity in Europe. Ecological studies, vol 167. Springer, Berlin
Oertli B (2010) The local species richness of Dragonfl ies in mountain waterbodies: an indicator of climate warming? In: Ott J (Ed) (2010) Monitoring Climatic Change With Dragonfl ies. BioRisk 5:243–251. https://doi.org/10.3897/biorisk.5.853
Ott J, Conze KJ, Günther A, Lohr M, Mauersberger R, Roland HJ, Suhling F (2021) : Rote Liste und Gesamtartenliste der Libellen (Odonata) Deutschlands. In: Ries M, Balzer S, Gruttke H, Haupt H, Hofbauer N, Ludwig G. & Matzke-Hajek G Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Band 5: Wirbellose Tiere (Teil 3). Münster (Landwirtschaftsverlag). Naturschutz und Biologische Vielfalt 70 (5):659–679
Raab R, Chovanec A, Pennerstorfer J (2006) Libellen Österreichs. Springer, Wien
Šenfeldr M, Kaczka R, Buras A, Smusevich A, Herrmann C, Spyt B, Menzel A, Treml V (2021) Diverging growth performance of co-occurring trees (Picea abies) and shrubs (Pinus mugo) at the treeline ecotone of Central European mountain ranges. Agric Forest Meteorology 308–309 (2021) 108608. https://doi.org/10.1016/j.agrformet.2021.108608
Wildermuth H (1999) Somatochlora alpestris (Selys, 1840) in den Schweizer Alpen: Eine Verbreitungs- und Habitatanalyse (Anisopera: Corduliidae). Odonatologica 28:399–416
Wildermuth H (2008) Die Falkenlibellen Europas. Corduliidae. Die Neue Brehm-Bücherei Bd.653. Westarp Wissenschaften, Hohen warsleben
Wildermuth H, Knaus P (2002) The impact of incidental summer snowfall on two alpine metapopulations of Somatochlora alpestris (Selys) (Anisoptera: Corduliidae). Odonatologica 31:55–6
Zeidler M, Fišerová E, Římalová V, Bednář M, Banaš M (2020) Sex-based differences in vigor and site preferences of Juniperus communis subsp. Nana Nordic J Botany 2020:e02812. https://doi.org/10.1111/njb.02812
Acknowledgements
We would like to thank Michal Hykel both for his help in the field and for valuable discusion of our findings, Marceau Minot (reviewer) for valuable comments and to OLS Brewery for inspiration.
Funding
Partial financial support for this work was recieved from Internal Grant Agency of Palacký University, Olomouc (IGAˍPrFˍ2022ˍ013).
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
Josef Kašák: investigation, conceptualization, methodology, writing – original draft preparation, writing – review and editing, supervision.
Otakar Holuša: conceptualization, methodology, writing – review and editing.
Monika Mazalová: investigation, writing – original draft preparation, writing – review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no financial or non-financial interests to disclose, neither any competing interest to declare that are relevant to the content of this article. The target species (Somatochlora alpestris) is not protected under the Nature and Landscape Protection Act of the Czech Republic (Act. No. 114/1992 Coll.). The data were collected outside any protected area within the meaning of the Act. No. 114/1992 Coll.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kašák, J., Holuša, O. & Mazalová, M. Artificial habitat – a chance for survival of a rare montane dragonfly (Odonata): case study on an alpine emerald (Somatochlora alpestris). J Insect Conserv 27, 315–321 (2023). https://doi.org/10.1007/s10841-023-00457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00457-z