Abstract
Over the last decades, numerous natural habitats have been converted into settlement areas, agricultural land, and tree plantations on a large spatial scale. As a result, natural ecosystems have been destroyed. In consequence, many ecosystems exist today as small and geographically isolated remnants. To what extent the original species diversity can persist in such small habitat patches is questionable and strongly depends on the ecology of the species. A prominent example of severe habitat destruction are the species-rich tropical cloud forests of Taita Hills in southern Kenya, which have been deforested almost completely during past decades. However, there still exist typical forest species in the few remaining forest fragments. In this study, we investigate the population ecology and behaviour of two butterfly species present in the cloud forest remnants of Taita Hills, Protogoniomorpha parhassus and Precis tugela. Over a period of one month, we conducted Mark-Release-Recapture to study population sizes and demographic structures, lifespan, dispersal, and behaviour. We found that both species exhibited medium population sizes and are sedentary. However, some individuals performed dispersal throughout the forest. The behaviour of the two species differs: While P. tugela was mostly observed basking with open wings, P. parhassus was mostly sitting under leaves with closed wings. The life span was rather long for butterflies.
Implications for insect conservation
This study documents the population ecology and behaviour of these two Afrotropical butterflies and underlines the relevance of the conservation of cloud forest remnants to preserve species, which mainly depends on these habitat remnants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transformation of still intact ecosystems into agricultural land has accelerated greatly over the last decades and has caused the destruction of habitats, particularly in the tropics (Bradshaw et al. 2009). Thus, numerous ecosystems today only exist as small and geographically isolated habitat remnants. As a consequence of these changes in habitat configuration, edge effects negatively impact habitat quality (Thomas 2016). Although simply being small remnants, they frequently host a large proportion of the original biodiversity. However, it is questionable whether species are able to persist in such small habitat remnants in the long term (Habel and Schmitt 2018).
Whether a species is able to persist under these conditions of habitat fragmentation and habitat deterioration strongly depends on its ecological demands and behaviour, in particular on its degree of ecological specialisation, adaptability, and dispersal (Thomas 2016). It has been shown that species with specific habitat requirements, comparatively narrow ecological variability, and little adaptability are suffering greatly under environmental changes, such as habitat fragmentation and reduction of habitat quality (Mangels et al. 2017). This means that dispersal ability and behaviour are pivotal for species’ survival, especially when they formerly existed in interconnected ecosystems (Ehl et al. 2019). In this context, mobile species are assumed to cope much better with habitat fragmentation than sedentary and highly specialized taxa do (Thomas 2016). Additionally, the life span of individuals also has a strong effect on the respective species’ persistence in a region or habitat because species with a longer life span of their individuals can bridge poor environmental conditions better than those with short-lived individuals, such as many insect species (Morris et al. 2008, Rodriguez-Caro et al. 2021).
The cloud forests of Taita Hills in southern Kenya represent one of these cases of dramatic losses of natural tropical forests with extraordinarily high diversity (Pellikka et al. 2009, 2013; Teucher et al. 2020). The Taita Hills cloud forests are one of the world’s biodiversity hotspots (Myers et al. 2000). Originally, cloud forests covered the entire higher elevations of this mountain ridge. However, almost all of these species-rich forests have been transformed into tree plantations composed of exotic species (such as Eucalyptus, Grevillea robusta, Cupressus lusitanica) or converted into arable land for subsistence agriculture (Teucher et al. 2020). Most of these transformations occurred within the last century (Pellikka et al. 2009). As a result, only few remnants of the species-rich cloud forest remain today, mostly on mountain peaks and rugged slopes with limited accessibility. These small forest pockets still hold a remarkable flora and fauna, some of which are endemic to the Taita Hills (Brooks et al. 1998).
While numerous studies have intensively investigated the species composition of animal and plant species, also considering habitat quality and invasive species, dedicated studies on population ecology and behavioural biology of specific species are still underrepresented, especially for tropical ecosystems. In this study, we selected butterflies as study organisms to analyse their population structure and behaviour. Butterfly species are excellent model organisms as most of them respond to and are highly sensitive to environmental changes (Bonebrake et al. 2010, Rákosy and Schmitt 2011). This is because they often require specific larval food plants and (micro)habitat structures as imagoes, as well as particular microhabitats for the developing larvae. Consequently, butterflies are also often used as indicators to evaluate the status of an ecosystem and/or of entire landscapes (Fleishman and Murphy 2009). Here we studied the population ecology and behaviour of butterflies. For this we used Mark-Release-Recapture (MRR), a powerful tool to analyse population sizes, demographic structures (including sex and age), and the behaviour of the individuals including dispersal (e.g., Ehl et al. 2018). Such information is highly suitable and reliable for the evaluation of species’ persistence in small and isolated habitats and for the development of efficient nature conservation measures.
For our MRR study we selected two nymphalid butterflies, the forest mother-of-pearl Protogoniomorpha parhassus (Drury 1782) and the African leaf butterfly Precis tugela (Trimen 1879). Both species occur in high densities in Taita Hills and can be observed mainly in and around the cloud forest patches and adjoining gardens (Kioko et al. 2021). Both species are rather large butterflies (with wing-spans of 75–100 mm for P. parhassus and 55–64 mm for P. tugela) and do not show sexual dimorphisms. These butterfly species are common, distributed across major parts of Subsahara Africa (Larsen 1991, 2005), and show comparatively strong dispersal behaviour (Kioko et al. 2021). They are mainly found in forest and woody habitats (Larsen 1991, 2005; Kioko et al. 2021). Their larvae feed on a variety of plants of the understorey (Larsen 1991, 2005). In addition, P. parhassus belongs to the species whose imagoes at least occasionally feed on rotting fruits (Molleman et al. 2006); their perching behaviour is reducing with increasing habitat degradation and perching sites are more quickly reoccupied in degraded than in non-degraded forest fragments (Bonte and van Dyck 2009).
Here, we address the following research questions:
-
1.
What is the population size and demographic structure of the local occurrence of the two species?
-
2.
What is the dispersal and behaviour of these two species, and do they differ in these aspects?
-
3.
What conclusions can be drawn from these findings for practical conservation, especially in context of ongoing forest destruction and the deterioration of habitat quality?
Material and methods
Study site
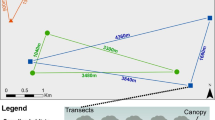
Study sites were selected in the Chawia forest fragment of Taita Hills (3° 29′ 06′′ S, 38° 20′ 45′′ E), where both species still occur in high densities (Schmitt et al. 2020; Kioko et al. 2021). This forest fragment covers an area of 86 ha of cloud forest and is located at the southern edge of Taita Hills, at an elevation of 1400–1600 m a.s.l. (highest point: 1607 m) (Fig. 1). Chawia forest is not gazetted under the forest conservation and management act of 2016, and thus is a community forest managed by the community forest associations (Himberg et al. 2009; Teucher et al. 2020). In consequence, this forest remnant suffers from diverse human activities and disturbances such as a dense network of roads and paths, collection of dead wood, planting of exotic tree species, infrastructure programmes (damming of small streams, affecting the hydrology of the forest), and hunting of animals (e.g. monkeys) (Rülke et al. 2020; Nzau et al. 2022). Thus, the Chawia forest fragment represents a highly disturbed cloud forest patch (see Pellikka et al. 2009). Nevertheless, the forest is still largely characterised by original cloud forest vegetation. It is surrounded by fields for subsistence agriculture (mostly annual maize and interspersed perennial banana cultivation) and tree plantations of mainly exotic and fast-growing tree species such as Eucalyptus spp., Grevillea robusta, Cupressus lusitanica (Pellikka et al. 2013).
Mark-release-recapture
MRR was conducted along already existing paths running along the forest edge and throughout the forest interior (in total about 3.5 km) (Fig. 1). The observation trail was walked at slow pace once a day (between 9:00 h and 16:00 h) from 2 to 30 September 2021, under largely sunny weather conditions and temperatures of at least 20 °C. Later, from 1 to 30 October non-systematic MRR was continued in the area, but data were only included in the calculation of dispersal distances and the longest life-span of individuals observed. Encountered individuals of P. parhassus and P. tugela were observed and captured with a butterfly net. We determined sex (male/female) and age according to wing condition considering the following three categories: 1 (completely undamaged wings including all marginal cilia), 2 (undamaged wings but without cilia), 3 (wings with moderate to heavily damaged wings) (see Zimmermann et al. 2005; Wendt et al. 2021). Individuals were then marked with a consecutive number at the underside of the hind wing using a waterproof pen and then released. For each marked butterfly, we noted date and time of capture, as well as respective weather conditions. Its exact location was determined using a GPS device (GARMIN GPSMap 64). Recaptures were treated similarly but labelled with “R” (= recapture) in our field book. In addition, the observed behaviour of each individual prior to capture was noted. Here we used the following categories: Flying, basking with open wings, resting with closed wings, or nectaring.
Statistics
The demographic structure was analysed under an open population formulation of the Jolly-Seber model, allowing entries and exits of the individuals to and from the population (POPAN model; Schwarz and Arnason 1996). The model estimates the following parameters: probability of an individual to enter the population (pent), survival probability (φ), capture probability (p), and gross population size (N). The specimen’s sex was treated as individual covariate. The parameters φ, pent, and N were tested for their dependence on sex by fitting multiple models with all combinations of sex-dependent and -independent parameters. φ, p, and pent were additionally tested for linear time dependence. Quadratic dependence on time and interactions of sex and time were tested for φ, and pent (a full list of models is given in Online Appendix 1). In total, we ran 144 models for each of the two species (6 φ × 6 pent × 2 p × 2 N). We chose the model that fitted our data best by comparing corrected AIC scores. All analyses were conducted in RMark v.2.2.7 (Laake 2013), which served as a frontend to software Mark (Linux v. 9.0, White and Burnham 1999).
We tested for differences in mean dispersal distances between females and males per species with two-sided t-tests in R (v. 4.2.0; R Core Team 2022). Distance measures between one and a subsequent capture locality of the same specimen were therefore log-transformed. Furthermore, the travelled distances were divided into distance classes (20 m, 30 m, and 50 m intervals, respectively), separately for each species and sex. To check for any potential artefacts caused by the chosen interval sizes, we analysed and compared these three interval size classes. The inverse cumulative percentage of these classes was determined, which corresponds to the probability density function, i.e. the dispersal kernel.
Based on these classes, the probabilities of dispersal flights were assessed by distance extrapolation. We used two frequently applied regression analyses, the negative exponential function (NEF) and the inverse power function (IPF). NEF tends to underestimate rare long-distance movements, whereas the IPF may encounter problems with ‘zero’ movements. The data were linearly transformed with a semi-ln plot for the NEF analyses or with a double-ln plot for the IPF analyses. In both equations, ‘P’ stands for the proportional probability that an individual will travel at least as far as the distance D, and ‘a’ for the intercept of the regression. NEF operates with the dispersal constant K as slope, whereas IPF uses the variable n as slope, which represents the effect of distance on dispersal (Ehl et al. 2018).
We selected the best model and the most suitable interval size, based on calculated stability indices (i.e. R2) of the calculated curves, which corresponds to the proportion of explained variance of the dependent variable by the independent variable. This allowed extrapolations of the population’s proportion that should travel distances exceeding the extent of the study area. The calculations were performed separately for males and females.
Results
We captured a total of 293 individuals of P. parhassus (77 females; 216 males) and 293 individuals of P. tugela (160 females; 133 males) (raw data are given in Online Appendix 2 and 3). Eleven females (14.3%) and 38 males (17.6%) of P. parhassus as well as 14 females (8.8%) and 16 males (12.0%) of P. tugela were recaptured at least once. The overall recapture rate was 13.5% (Table 1).
In both species, the models that fitted the data best suggested the entrance probability pent to decrease over time (linear estimate in P. parhassus, quadratic in P. tugela; see Online Appendix 1). While no sex-dependence of any parameter was estimated for P. tugela, the parameters population size N and survival rate φ differed among sexes in P. parhassus. In contrast to P. tugela (607 females ± 90 SE; 610 males ± 90 SE), a strong excess in males was estimated for P. parhassus (205 females ± 23 SE; 793 males ± 108 SE). Mean minimum ages (time difference between first and last capture) were slightly higher in males of both species. Maximum ages were 23 days and 26 days for P. tugela and P. parhassus, respectively. Daily survival rates of P. parhassus varied over time (estimate quadratic; median females 1.00 ± 0.02; males 1.00 ± 0.06). Median daily survival rates of P. tugela were 0.91 ± 0.01, irrespective of sex (see Fig. 2 ). The age structure assessed by wing conditions did not change remarkably over time (Fig. 3).
Individuals largely remained in the same area throughout the study period. Individuals of P. tugela were generally recaptured farther away from their place of capture (female median = 33.9 m; male median 85.7 m) than individuals of P. parhassus (female median = 20.1 m; male median 27.1 m) (Fig. 4). Males tended to higher mobility in both species. However, no significant differences were found in any comparison. Few individuals dispersed exceptionally far (identified as outliers in box-whisker plots, i.e., deviating more than 1.5 times the interquartile range from the upper quartile). While both sexes showed such disperser individuals, they were more frequent and more extreme in males of both species (maximum distances P. tugela: 1,368 m; P. parhassus: 1,372 m) than in females (maximum distances P. tugela: 721 m; P. parhassus: 675 m).
We applied the NEF and IPF functions for extrapolating the potential for long-distance dispersal. IPF returned higher stability indices (R2) in all pairs of functions. In all cases, explained variance was higher in males (range R2 values based on IPF: 0.94–0.97) than in females (0.79–0.90); consequently, estimates for males should be more reliable than for females. R2 values within both functions were quite similar for the three different distance classes within sexes and within species (Table 2). So, as no relevant differences existed between these classes, only the results for the 30 m intervals (as the intermediate one) are given below; all results are given in Online Appendix 2 and 3.
Extrapolations for 1 km were roughly similar for both functions within both species but estimates declined much stronger for NEF than for IPF with longer distances. Thus, following NEF, longer dispersal events would be little likely for both species, while they are expected under the IPF model. In general, following the better supported IPF model, the likelihood of reaching a defined distance was twice as high for P. tugela than for P. parhassus. Thus, about 10% of P. tugela males and females should reach distances of 1 km or more, and still 3 to 4% should travel 5 km or more. No difference in dispersal behaviour was obtained between sexes. For P. parhassuss, about 5.1% of males and 6.4% of females are assumed to disperse at least 1 km. With distances of 5 km or more, these values decreased to 1.3% (males) and 2.2% (females). Thus, the slightly female-biased dispersal in P. parhassus seems to increase with distance (Table 3, Fig. 4). A Spearman rank correlation did not reveal a correlation between dispersal distance and the elapsed time between capture and first recaptures for females or males.
P. tugela and P. parhassus did not show markedly different behaviour in terms of flight, interaction, or feeding. A conspicuous difference, however, was observed for the resting behaviour. While P. parhassus was mainly observed resting with closed wings, P. tugela rested mainly with open wings (Fig. 5).
Discussion
Population size and demography
Population sizes and respective densities estimated for both study species are largely consistent with results from previous MRR studies of tropical forest butterflies (e.g. Marín et al. 2009; Vlasanek et al. 2013; Vlasanek and Novotny 2015; Seixas et al. 2017). Only few of these so far studied species exhibited remarkably higher densities than in our study (e.g. Marín et al. 2009; Vlasanek et al. 2013; Vlasanek and Novotny 2015). Butterfly species, which occur predominantly in forests and woody landscapes, show similar values, such as Euphydryas maturna in Czech Republic (Konvicka et al. 2005). In tropical forests, however, butterflies can also accumulate at very high densities at certain locations, such as around watering holes, spots with high numbers of blossoms, locations with overripe fruits (Habel et al. 2022), as well as in forest gaps (Hill et al. 2001). This would also apply to P. parhassus, which can be found in high densities at watering places and feasting on overripe fruits (Molleman et al. 2006).
The medium sized population densities found for these two species contrasts with the sometimes very high densities of species, which occur restricted to very specific habitat types such as bogs and wetlands, or in various types of grassland ecosystems (Junker and Schmitt 2010; Zimmermann et al. 2011; Weyer and Schmitt 2013; Ehl et al. 2017, 2018). Such high population densities are rarely reached by butterflies predominantly found in forests and wooded ecosystems. These differences in butterfly densities might be due to the spatial distribution of resources, which are rather homogenously distributed across forested areas (Mbuthia 2003). Consequently, the butterflies might be less abundant and are more widely distributed across the entire ecosystem and landscape. However, the comparatively low population densities found here might also reflect a methodological artefact: Since in forest observers are largely bound to walk along existing pathways (and thus only cover a limited proportion of the ecosystem, also excluding the highly relevant canopy ecosystem), observers may cover the entire area of a butterfly’s habitat in open ecosystems and thus may detect a much larger fraction of the individuals which are present.
Another factor shaping population densities are shifting environmental conditions. In temperate regions, life spans of butterfly individuals as imagoes are mostly restricted to some days to 2–3 weeks (e.g. Zimmermann et al. 2011; Pennekamp et al. 2014; Ehl et al. 2017). Hence, they build up spatially and temporally constricted populations (e.g. Fric et al. 2010; Junker and Schmitt 2010; Weyer and Schmitt 2013). Similarly, arthropods of the arid and semi-arid tropical ecosystems also follow annual population cycles, triggered by dry and rainy seasons. These seasonal shifts frequently result in strong fluctuations in population densities (see Habel et al. 2018; Schmitt et al. 2021). However, most butterfly species of wet tropical forest ecosystems occur year-round and form much less or even no distinct generations, in contrast to species of temperate regions (Molleman et al. 2007; Seixas et al. 2017). Furthermore, they are often much more long-lived (see Beck & Fiedler 2009). Studies on Afrotropical butterflies in western Uganda showed comparatively long life spans (mean 39 days) and a longevity record of 230 days for Charaxes fulvescens (Molleman et al. 2007). This trend corresponds with our own results for both, P. parhassus and P. tugela, showing rather long life-times (including a longevity record of 52 days for P. parhassus).
Interestingly, our data analyses yield a higher population size for P. parhassus males than for females. We assume this difference in our analyses being an artefact resulting from the much higher number of marked males in combination with lower recapture rates in females (14.3%) than in males (17.6%). This apparently has led to lower female population size estimates and a generally worse coverage of females. This contradicts the results of breeding experiments, which generally showed a balanced sex-ratio for butterflies (Ashoff and Schmitt 2014; Seixas et al. 2017). The observed imbalance in sex ratios could be due to several reasons: 1. For other butterfly species, females show a rather cryptic behaviour while males actively patrol and thus are more visible (Seixas et al. 2017, with references therein); distinct patrolling behaviour has already been demonstrated for P. parhassus, especially in disturbed forest fragments of Taita Hills (see Bonte and Dyck 2009). 2. Females may be more abundant in the high canopies and thus less likely to encounter observers on the ground. 3. Population dynamics could be subject to protandry (at the beginning of the generation males are much more abundant than females, gaining dominance only from the second half of the generation period, e.g. Junker and Schmitt 2010; Weyer and Schmitt 2013; Pennekamp et al. 2014; Ehl et al. 2019); however, such protandry is the exception for tropical butterflies, and our results also show neither a clear temporal population dynamic nor significant changes in sex ratios over time. Further studies are essential to clarify these contingencies.
Mobility and behaviour
Our data provide important insights into the mobility behaviour of the two studied species. Most marked and recaptured individuals were sedentary and remained close to their location of first capture. Most individuals did not move farther than 100 m, i.e. P. parhassus males: 72%, females: 87%; P. tugela males: 69%, females: 65%. This finding is consistent with the results from numerous other MRR studies on butterflies, from the tropics and the temperate regions (Konvicka et al. 2008, Fric et al. 2010, Vlasanek et al. 2013, Vlasnek and Nowotny 2015, Seixas et al. 2017, Ehl et al. 2018, Wendt et al. 2021). Most individuals of the studied non-migratory butterfly species remained close to their point of capture with fairly restricted movements except for some few individuals performing long distance dispersal (Lewis 2001, Marín et al. 2009, Vlasanek et al. 2013, Vlasnek and Nowotny 2015, Seixas et al. 2017).
A few individuals in our study also dispersed throughout the forest, with maximum distances of 1,372 m (P. parhassus) and 1,368 m (P. tugela). However, extrapolation of data calls for considerably higher dispersal capacity of the more generalist P. tugela than in P. parhassus, being more strongly linked to forests. Compared to many other butterfly species (e.g. Konvicka et al. 2005, 2008; Zimmermann et al. 2005, 2011; Fric et al. 2010; Junker and Schmitt 2010; Junker et al. 2010; Weyer and Schmitt 2013; Ehl et al. 2017, 2018, 2019; Wendt et al. 2021), and in contrast to the sedentary behaviour of the majority of the individuals assessed in our study, the proportion of P. parhassus individuals dispersing over distances of kilometres is high and even very high in P. tugela. Long-distance dispersal even across non-habitat areas therefore seems likely for the less specialized species P. tugela. This species therefore might interconnect its populations all over the range of Taita Hills by dispersal. In case of a local extinction, recolonization should be realised quickly for this species. In general, such long-distance movements (even if they rarely take place) are highly relevant, as they enable habitat (re)colonization and ensure the exchange of individuals among single forest fragments. These dispersal events create the pre-condition for long-term persistence of local populations. The lower dispersal power and stronger habitat link of P. parhassus makes this scenario less likely in this second case, making exchange between forest fragments and recolonization considerably less likely for this species.
Because we captured and marked butterflies in this study exclusively along a defined path within and at the immediate edge of the forest, it is possible that movement distances were clearly underestimated. This is a general problem that occurs in MRR studies, since trapping is usually only done where there is a high probability of capturing many individuals of a particular species. Thus, the situation arises that only within the habitat, and rarely outside in the landscape matrix, data are collected—and thus movement distances are generally significantly underestimated.
We also found that the two studied species differed in their behaviour. While P. parhassus can be observed sitting under/on vegetation with closed wings inside the shady forest, (especially under large leaves of various shrub and tree species), P. tugela spends a lot of time basking in the sun with open wings. This butterfly species was much more prevalent along the sunny forest edges as well as in adjacent gardens and shrub structures in the agricultural landscape (Schmitt et al. 2020, own observations JCH, TS). Thus, the two species can be clearly distinguished from each other with respect of their ecological demands and behaviour. P. parhassus is a typical species of humid forests and thus occurs mostly in shady areas in the forest of our study area where it mostly shows a sedentary behaviour. On the contrary, P. tugela is more mobile and flies longer distances, often encountered beyond the forest (Schmitt et al. 2020), making it a less typical forest species with a variety of alternative habitats (Larsen 1991; Kioko et al. 2021).
To get a more realistic picture of the population ecology, dispersal and behaviour of these two butterfly species, additional studies with further methods would be useful. For example, larger areas could be equipped with bait traps in order to record larger movement distances of the butterflies. In addition, this method could be used to attract more butterflies and thus significantly increase the sample size. Thus, data on density and movement distance would become more reliable. Here, attention should also be paid to longer data recording in order to be able to make better statements about the lifespan of the butterflies. However, the results assessed with bait traps could also provide various shortcomings which has to be taken into consideration while interpreting the outcomes. For example, butterflies would be actively attracted with bait traps and thus might bridge greater distances than they do on average (Habel et al. 2022).
Conservation consequences
Our study shows that most individuals of both butterfly species (with a few exceptions) are site-faithful. P. parhassus occurs in restricted areas and seems to depend on cloud forest (see also Schmitt et al. 2020). The estimated dispersal behaviour of this species suggests that an exchange of individuals between the existing forest fragments is unlikely due to the geographic distance between fragments, and even dispersal via plantations with cloud forest elements might be difficult to cross. This might be different for P. tugela because of its higher dispersal capacity and its lower habitat specialisation (Schmitt et al. 2020). Nevertheless, strict protection and reconnection of the cloud forest remnants and increasing the sizes of forest fragments would be an important conservation measure (see Aben et al. 2016), which may help to increase population and species persistence, especially of forest species. The removal of Eucalyptus trees (and other alien plant species) would also be an important measure to restore this disturbed and degraded forest. This might increase habitat quality and support survival probability of all species living there.
References
Aben J, Bocedi G, Palmer SCF et al (2016) The importance of realistic dispersal models in conservation planning: application of a novel modelling platform to evaluate management scenarios in an Afrotropical biodiversity hotspot. J Appl Ecol 53:1055–1065
Ashoff M, Schmitt T (2014) Are different allozyme genotypes of the butterfly Polyommatus coridon adapted to resist cold and heat shocks? Ann Zool Fenn 51:413–422
Beck J, Fiedler K (2009) Adult life spans of butterflies (Lepidoptera: Papilionoidea + Hesperioidea): broadscale contingencies with adult and larval traits in multi-species comparisons. Biol J Linn Soc 96:166–184
Bonebrake TC, Ponisio LC, Boggs CL, Ehrlich PR (2010) More than just indicators: a review of tropical butterfly ecology and conservation. Biol Conserv 143:1831–1841
Bonte D, Van Dyck H (2009) Mate-locating behaviour, habitat-use, and flight morphology relative to rainforest disturbance in an Afrotropical butterfly. Biol J Linn Soc 96:830–839
Bradshaw CJ, Sodhi NS, Brook BW (2009) Tropical turmoil: a biodiversity tragedy in progress. Front Ecol Env 7:79–87
Brooks T, Lens L, Barnes J et al (1998) The conservation status of the forest birds of the Taita Hills, Kenya. Bird Conserv Int 8:119–139
Ehl S, Ebertshäuser M, Gros P, Schmitt T (2017) Population demography of alpine butterflies: Boloria pales and Boloria napaea (Lepidoptera: Nymphalidae) and their specific adaptations to high mountain environments. Acta Oecol 85:53–61
Ehl S, Dalstein V, Tull F et al (2018) Specialized or opportunistic-how does the high mountain endemic butterfly Erebia nivalis survive in its extreme habitats? Population ecology of Erebia nivalis. Insect Sci 25:161–171
Ehl S, Böhm N, Wörner M et al (2019) Dispersal and adaptation strategies of the high mountain butterfly Boloria pales in the Romanian Carpathians. Front Zool 16:1
Fleishman E, Murphy DD (2009) A realistic assessment of the indicator potential of butterflies and other charismatic taxonomic groups. Conserv Biol 23:1109–1116
Fric Z, Hula V, Klimova M et al (2010) Dispersal of four fritillary butterflies within identical landscape. Ecol Res 25:543–552
Habel JC, Schmitt T (2018) Vanishing of the common species: empty habitats and the role of genetic diversity. Biol Conserv 218:211–216
Habel JC, Seibold S, Ulrich W, Schmitt T (2018) Seasonality overrides differences in butterfly species composition between natural and anthropogenic forest habitats. Anim Conserv 21:405–413
Habel JC, Ulrich W, Eberle J, Schmitt T (2022) Species community structures of Afrotropical butterflies differ depending on the monitoring method. Biodiv Conserv 31:245–259
Hill J, Hamer K, Tangah J, Dawood M (2001) Ecology of tropical butterflies in rainforest gaps. Oecologia 128:294–302
Himberg N, Omoro L, Pellikka P, Luukkanen O (2009) The benefits and constraints of participation in forest management. The case of Taita Hills. Kenya Fennia Int J Geogr 187:61–76
Junker M, Schmitt T (2010) Demography, dispersal and movement pattern of Euphydryas aurinia (Lepidoptera: Nymphalidae) at the Iberian Peninsula: an alarming example in an increasingly fragmented landscape? J Insect Conserv 14:237–246
Junker M, Wagner S, Gros P, Schmitt T (2010) Changing demography and dispersal behaviour: ecological adaptations in an alpine butterfly. Oecologia 164:971–980
Kioko EN, Musyoki AM, Luanga AE, et al. (2021) Fluttering beauty with benefits: The butterflies of Taita Hills. A field guide. National Museums of Kenya, Nairobi, Kenya.
Konvicka M, Čížek O, Filipová L et al (2005) For whom the bells toll: demography of the last population of the butterfly Euphydryas maturna in the Czech Republic. Biologia 60:551–557
Konvicka M, Novak J, Benes J et al (2008) The last population of the woodland brown butterfly (Lopinga achine) in the Czech Republic: habitat use, demography and site management. J Insect Conserv 12:549–560
Larsen TB (1991) The butterflies of Kenya and their natural history. Oxford University Press, Oxford
Larsen TB (2005) Butterflies of West Africa, Apollo Books, 595 + 270 pp.
Laake J (2013) RMark: An R Interface for Analysis of Capture-Recapture Data with MARK. AFSC Processed Rep 2013-01, 25 p. Alaska Fish Sci Cent, NOAA, Natl Mar Fish Serv, Seattle, WA.
Lewis OT (2001) Effect of experimental selective logging on tropical butterflies. Conserv Biol 15:389–400
Mangels J, Fiedler K, Schneider FD, Blüthgen N (2017) Diversity and trait composition of moths respond to land-use intensification in grasslands: generalists replace specialists. Biodiv Conserv 26:3385–3405
Marín L, León-Cortés JL, Stefanescu C (2009) The effect of an agro-pasture landscape on diversity and migration patterns of frugivorous butterflies in Chiapas, Mexico. Biodiv Conserv 18:919–934
Mbuthia KW (2003) Ecological and ethnobotanical analyses for forest restoration in the Taita Hills. Miami University, Kenya
Molleman F, Kop A, Brakefield PM, Zwaan BJ (2006) Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodiv Conserv 15:107–121
Molleman F, Zwaan B, Brakefield P, Carey J (2007) Extraordinary long life spans in fruit-feeding butterflies can provide window on evolution of life span and aging. Exp Gerontol 42:472–482
Morris WF, Pfister CA, Tuljapurkar S et al (2008) Longevity can buffer plant and animal populations against changing climatic variability. Ecology 89:19–25
Myers N, Mittermeier RA, Mittermeier CG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nzau JM, Ulrich W, Rieckmann M, Habel JC (2022) The need for local-adjusted participatory forest management in biodiversity hotspots. Biodiv Conserv 31:1313–1328
Pellikka PKE, Lötjönen M, Siljander M, Lens L (2009) Airborne remote sensing of spatiotemporal change (1955–2004) in indigenous and exotic forest cover in the Taita Hills, Kenya. Int J Appl Earth Obs Geoinf 11:221–232
Pellikka PKE, Clark BJF, Gosa AG et al (2013) Agricultural expansion and its consequences in the Taita Hills. Developments in earth surface processes. Elsevier, Amsterdam, pp 165–179
Pennekamp F, Garcia-Pereira P, Schmitt T (2014) Habitat requirements and dispersal ability of the Spanish Fritillary (Euphydryas desfontainii) in southern Portugal: evidence-based conservation suggestions for an endangered taxon. J Insect Conserv 18:497–508
Rákosy L, Schmitt T (2011) Are butterflies and moths suitable ecological indicator systems for restoration measures of semi-natural calcareous grassland habitats? Ecol Ind 11:1040–1045
Rodríguez-Caro RC, Capdevila P, Graciá E et al (2021) The limits of demographic buffering in coping with environmental variation. Oikos 130:1346–1358
Rülke J, Rieckmann M, Nzau JM, Teucher M (2020) How ecocentrism and anthropocentrism influence human-environment relationships in a Kenyan biodiversity hotspot. Sustainability 12:8213
Schmitt T, Ulrich W, Büschel H et al (2020) The relevance of cloud forest fragments and their transition zones for butterfly conservation in Taita Hills, Kenya. Biodiv Conserv 29:3191–3207
Schmitt T, Ulrich W, Delic A et al (2021) Seasonality and landscape characteristics impact species community structure and temporal dynamics of East African butterflies. Sci Rep 11:15103
Schwarz CJ, Arnason AN (1996) A General methodology for the analysis of capture-recapture experiments in open populations. Biometrics 52:860
Seixas RR, Santos SE, Okada Y, Freitas AVL (2017) Population Biology of the sand forest specialist butterfly Heliconius hermathena hermathena (Hewitson) (Nymphalidae: Heliconiinae) in Central Amazonia). J Lepid Soc 71:133–140
Teucher M, Schmitt CB, Wiese A et al (2020) Behind the fog: Forest degradation despite logging bans in an East African cloud forest. Global Ecol Conserv 22:e01024
Thomas JA (2016) Butterfly communities under threat. Science 353:216–218
Vlasanek P, Sam L, Novotny V (2013) Dispersal of butterflies in a New Guinea rainforest: using mark–recapture methods in a large, homogeneous habitat. Ecol Entomol 38:560–569
Vlasanek P, Novotny V (2015) Demography and mobility of three common understory butterfly species from tropical rain forest of Papua New Guinea. Pop Ecol 57:445–455
Wendt M, Senftleben N, Gros P, Schmitt T (2021) Coping with environmental extremes: Population ecology and behavioural adaptation of Erebia pronoe, an alpine butterfly species. InSects 12:896
Weyer J, Schmitt T (2013) Knowing the way home: strong philopatry of a highly mobile insect species, Brenthis ino. J Insect Conserv 17:1197–1208
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals”. Bird Study 46:120–138
Zimmermann K, Fric Z, Filipová L, Konvička M (2005) Adult demography, dispersal and behaviour of Brenthis ino (Lepidoptera: Nymphalidae): how to be a successful wetland butterfly. Eur J Entomol 102:699–706
Zimmermann K, Fric Z, Jiskra P et al (2011) Mark-recapture on large spatial scale reveals long distance dispersal in the Marsh Fritillary, Euphydryas aurinia. Ecol Entomol 36:499–510
Acknowledgements
We thank Tobias Seifert (Salzburg, Austria) and Amos Mwamburi (Kenya) for field assistance and data collection. We thank the German Academic Exchange Service for funding (programme Partnerships for Supporting Biodiversity in Developing Countries, project Biocult). We are grateful for critical and fruitful comments by two anonymous reviewers on a draft version of this article.
Funding
German Academic Exchange Service (programme Partnerships for Supporting Biodiversity in Developing Countries, project Biocult) financed travelling and field work in Kenya. Open access funding provided by Paris Lodron University of Salzburg.
Author information
Authors and Affiliations
Contributions
JCH and TS designed the study, JC collected the data in the field, JE performed the analyses. All authors contributed while writing
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habel, J.C., Eberle, J., Charo, J. et al. Population ecology and behaviour of two Afrotropical forest butterflies. J Insect Conserv 27, 271–281 (2023). https://doi.org/10.1007/s10841-022-00451-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00451-x