Abstract
Recent studies reporting widespread declines in arthropod biomass, abundance and species diversity raised wide concerns in research and conservation. However, repeated arthropod surveys over long periods are rare, even though they are key for assessing the causes of the decline and for developing measures to halt the losses. We repeatedly sampled arthropod fauna in a representative Swiss agricultural landscape over 32 years (1987, 1997, 2019). Sampling included eight study sites in four different semi-natural and agricultural habitat types and different trap types (pitfall, window, yellow bucket) over an annual period of 10 weeks to capture flying and ground dwelling arthropod taxa. In total, we analyzed 58,448 individuals from 1343 different species. Mean arthropod biomass, abundance and species richness per trap was significantly higher in 2019 than in the prior years. Also, species diversity of the study area was highest in 2019. Three main factors likely have contributed to the observed positive or at least stable development. First, the implementation of agri-environmental schemes has improved habitat quality since 1993, 6 years after the first sampling. Second, landscape composition remained stable, and pesticide and fertilizer was constant over the study period. Third, climate warming might have favored the immigration and increase of warm adapted species. Our results support the idea that changes in arthropod communities over time is highly context-dependent and complex.
Implications for insect conservation
We conclude that the integration and long-term management of ecological compensation patches into a heterogenous agricultural landscape supports insect conservation and can contribute to stable or even increased arthropod abundance, biomass and diversity. Future studies are needed to clarify interdepending effects between agricultural management and climate change on insect communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In agroecosystems, arthropods provide important ecosystem functions and services such as pollination (Gallai et al. 2009), pest control (Bianchi et al. 2006) or nutrient cycling (Belovsky and Slade 2000). Furthermore, arthropods are an important resource for many insectivores (Bowler et al. 2019; Vaughan 1997). Recent long-term studies reporting dramatic insect declines all over the world raised wide concerns about cascading effects on the overall biodiversity. Such temporally synchronous developments on different trophic levels are indicated by strong declines in specialized insectivorous farmland birds over Europe (Bowler et al. 2019; Jenni and Graf 2018). In 2017, the entomological society of Krefeld reported a decline of 75% in flying insect biomass in German nature reserves over a period of 27 years (Hallmann et al. 2017). Over a period of 10 years, Seibold et al. (2019) measured similarly strong declines in insect biomass, abundance and species diversity of 67%, 78% and 34%, respectively in German grasslands. Studies have previously drawn attention to declining trends in abundance and diversity in Europe and North America (Dirzo et al. 2014) and this has been supported by many other even earlier and more recent studies on individual arthropod groups. For example, bees, hoverflies, butterflies and carabid beetles were observed to decline in species diversity (Bartomeus et al. 2018; Biesmeijer et al. 2006; Homburg et al. 2019; Maes and van Dyck 2001; Soroye et al. 2020) and abundance (Brooks et al. 2012; Schuch et al. 2012; Thomas et al. 2004). At the global scale, a decline of 9% per decade was assessed for terrestrial insects (van Klink et al. 2020). This would mean a halving of insect abundance over 75 years, which is suggested to cause substantial and irreplaceable losses of ecosystem services to humanity (Cardoso et al. 2020). Habitat loss and agricultural intensification with the resulting habitat degradation are seen as the most important reasons for insect declines (Cardoso et al. 2020). However, among studies investigating long term insect trends, highly modified landscapes like farmland are underrepresented (van Klink et al. 2020). There are strong indications from other regions like the tropics that climate change and in particular altered precipitation strongly contribute to declining insect trends (Wagner 2020). Other authors warned of increasing droughts as an additional cause of insect declines in non-tropical regions as well (Wagner et al. 2021b), although some positive biodiversity responses have been reported (Outhwaite et al. 2022). Several meta-analyses revealed large variations in local trends with even positive trends for certain (aquatic) insect groups and observation periods in particular regions (Crossley et al. 2020; Outhwaite et al. 2022; van Klink et al. 2020). For example, moth biodiversity trends varied considerably in a comparison including Ecuador, Arizona and Costa Rica (Wagner et al. 2021a) and tiger moth species richness even mostly increased on Barro Colorado Island in Panama (Lamarre et al. 2022). This all shows that changes in insect abundance and diversity are complex, heterogenous and do hardly correlate amongst taxa (van Klink et al. 2022). All studies revealed winners and losers among insect taxa (Jackson et al. 2022), but their proportion varied. Each study contributes therefore to the general body of knowledge about changes in species richness and abundance of arthropods in general.
The Swiss Limpach valley offered a unique opportunity for a repeated arthropod assessment spanning 32 years in an agricultural landscape. It is a representative agricultural landscape for Central Europe with temporary grassland, cereals, and maize as the main cultures and with only small semi-natural habitat patches left. In this landscape, the arthropod fauna was recorded in different habitat types with standardized methods in a large-scale field study in 1987 (Duelli and Obrist 2003). The study was then repeated 10 years later (Gygax 1999). In 2019, we resampled again in the same habitat types, at closest possible proximity (crop rotation) and with the same trapping methods, which allowed us to compare arthropod (insects and spiders) biomass, abundance and species richness of the years 1987, 1997 and 2019. We examined a majority of arthropod taxa and trophic guilds occurring in these habitats, namely all beetles (Coleoptera), true bugs (Hemiptera: Heteroptera), spiders (Araneae), aculeate bees, wasps and ants (Hymenoptera: Aculeata), hover flies (Diptera: Syrphidae), lacewings (Neuroptera), snakeflies (Raphidioptera) and scorpionflies (Mecoptera). In particular, we addressed the following research questions: (1) Are there changes in arthropod biomass, abundance or species richness in the agricultural Limpach valley since 1987 and 1997? (2) Are there differences in temporal changes between habitat types, taxa and trophic guilds?
Methods
Study area and sampling
Our study area, the Limpach valley, is located in the Swiss Central Plateau (47.10° N, 7.44° W, cantons BE and SO). It extends over 13 km along a flat, agricultural plain bordered by two hills at an altitude of around 470 m above sea level. Formerly a large swamp and peat-cutting landscape, it was drained and converted into intensively cultivated farmland mainly during the two World wars and until 1951 (Imhof 1988). Two semi-natural habitat patches are still left, a south-exposed, dry, low-fertility meadow bordering the forest and a wetland reserve (Wengimoos), the last remnant of the former swamp. Both patches were expanded through restoration measures since the first arthropod survey in 1987.
In 2019, we placed traps for sampling arthropods in four habitat types at eight different sites (two per habitat type) in a transect of 5 km length through the valley. Seven of these sites had already been sampled in the year 1987 (Duelli and Obrist 2003) (only one at dry meadow). Four were resampled in a partial repetition (one per habitat type) in 1997 (Gygax 1999). The spatial extent of data considered is identical in all 3 years of sampling, but the number of repetitions per habitat type varied. The sites covered four agricultural and four semi-natural habitat types: two temporary grasslands, two wheat fields, two dry, low-fertility meadows and two wet meadows in the wetland reserve (Fig. 1).
Study area with the eight study sites of 2019 depicted as white symbols. Black symbols indicate the seven sites from 1987, grey symbols show the four sites from 1997. Habitat types are illustrated by different symbols; triangle = wet meadow, circle = temporary grassland, square = wheat field, rhomboid = dry meadow. Study site positions are not exactly the same because of crop rotation among the years, but they did vary less than 300 m between the sampling years. Biodiversity promotion areas (BPA) of the year 2019 are drawn in orange (we have no data of the location of BPA’s in 1997 and the system did not yet exist in 1987). Inset: Location of the study area (circle) in Switzerland
In 2019 we used the same trap types as in the years 1987 and 1997. At each site, two pitfall funnel traps (15 cm diameter), a window trap (glass plate 50 cm × 83 cm, 1.2 m above ground) and a yellow bucket (20.5 cm diameter, 20 cm above the vegetation, maximum at 1 m height) were placed (Online Appendix S1). Additionally, a combi-trap was placed at each site in 1997 and 2019. It is a combination of the yellow bucket and window trap (2 plexiglass plates 50 cm × 41.5 cm crossed over a yellow funnel of 42 cm diameter, 1 m above ground) and showed comparable results to the sum of the single traps (Duelli et al. 1999; Gygax 1999). In 1997, five pitfall traps instead of 2 were placed at each site (Table 1). In the analyses, we only compared the same trap types with each other and took account of the uneven trap numbers by averaging over the traps. Traps at each site were placed at least 10 m away from the field edge and in 5 m distance to each other. All traps were filled with water and 0.2% Rocima GT antifouling detergent (Acima, Buchs SG, Switzerland) as preservative. Traps were emptied weekly. Pitfall traps catch ground-dwelling arthropods, whereas the other three trap types catch flying insects (flight traps). Window and pitfall traps catch moving arthropods randomly (Duelli et al. 1999), whereas the yellow bucket and the yellow part of the combi-trap attract flower visiting insects actively (Yi et al. 2012). Because the used trap types catch only moving arthropods, the results represent a mixed measure of population density and activity pattern, the so-called activity-density (Yi et al. 2012).
We started sampling 3 weeks after the dandelion full bloom (Taraxacum officinale; 90% of the flowers open), at the beginning of May. This phenological start time is used to calibrate for variation in spring weather and altitude above sea level (Duelli et al. 1999). To reduce sampling and species identification effort, we conducted the sampling over 10 weeks according to the optimized minimal sampling program opti3 + 2. According to Duelli et al. (1999) biodiversity assessments of an area with reduced sampling and species identification effort can be best achieved by sampling in two periods of five weeks with a break of 1 week in between. In the end, out of the 10 study weeks, the three and 2 weeks richest in individuals from the first and second study period respectively are analysed (Duelli et al. 1999). Possible effects of bad weather (rainy, cold) could thus be balanced out by picking only the vials of the weeks containing highest abundances across all sites. The financial effort of the opti3 + 2 program is 20% of a full season sampling, but still yields 49% of the species diversity and 37% of all individuals (Duelli et al. 1999). We selected the opti3 + 2 weeks separately for pitfall and flight traps (Online Appendix S2).
To compare landscape configuration since 1987 we calculated the area of forest cover, agricultural land and gardens, houses and roads in a buffer of 2 km around the study sites. We used historized map data from the Federal Office of Topography (swisstopo) for this purpose [swissTLM3D © 2020 swisstopo (5,704,000,000)].
Analyzed taxa
The following arthropod taxa were identified to the species level: Araneae, Coleoptera, Diptera (only Syrphidae), Hymenoptera (only Aculeata incl. Formicidae), Hemiptera: Heteroptera, Mecoptera, Neuroptera and Raphidioptera. These taxa were selected because they cover a majority of the arthropods sampled (50–80%) and represent a wide range of trophic guilds. Individuals that could not be identified to the species level were excluded from the analysis. To ensure that no bias was introduced by excluding these individuals, taxa with an insufficient identification resolution (less than 80% of the individuals from the taxon were identified to species level), the family, subfamily or genus were excluded from the analysis (Online Appendix S3). However, for the Curculionidae we decided to make an exception, because the identification proportion was too low only for the year 1997. Keeping the Curculionidae in the analysis did not change the observed differences. Excluded taxa are the coleopteran families Cryptophagidae, Coccinellidae, Latridiidae, Melyridae, Dermestidae, Mordellidae, Nitidulidae, Ptiliidae, Phalacridae, Scraptiidae and Chrysomelidae, further the staphylinid subfamilies Aleocharinae and Pselaphinae as well as the heteropteran families Anthocoridae and Nabidae. Taxa with a clustered occurrence like the honeybee (Apis mellifera) and carrion beetles (Silphidae), which were attracted by the smell of carrion, were also excluded from the analysis.
Classification into trophic guilds
All species were classified into one out of six trophic guilds according to their main food source (herbivore, carnivore, detritivore, fungivore, trophobiotic, omnivore). If a species used different food sources to a similar extent across larval and adult stage, it was classified as omnivore (Gossner et al. 2015).
Biomass calculation
We calculated biomass using the weight-length relationship determined by Sohlström et al. (2018):
Intercept a and slope b were chosen individually for each taxon (from Model 5, Supporting Information 1 in Sohlström et al. 2018). Information on body length of each species was obtained from existing databases or compiled from the literature.
Data analysis
For analyses, the freshly sampled records were merged with the extracted data from the historic samples, which are stored in a WSL internal database (https://doi.org/10.16904/envidat.163). Analyses were performed using the Software RStudio version 2021.09.1 with the underlying R engine (R Core Team 2021). For each trap we compiled the species-specific count data into weekly biomass, abundance and number of species values. Pitfall and combi-traps were analyzed separately, whereas we pooled the weekly data of the yellow bucket and window trap per site and analyzed it as yellow + window traps. The pooling has two advantages: firstly, the results are better comparable with the results of the combi-traps (Gygax 1999) and secondly, we can avoid small samples, because the yellow bucket traps often caught only small amounts of insects. Sample sizes for each year can be calculated by: Number of traps per site*sites*5 weeks.
Weather data (mean daily air temperature [°C] 2 m above ground, mean daily wind speed [m/s], daily precipitation sum [mm]) were obtained from the nearest MeteoSwiss weather station, located 15 km east of the study area. We calculated mean weather values for all opti3 + 2 study weeks. In addition, we also calculated climate variables of previous years to consider potential lag-effects, number of frost days of the previous winter as well as 5-year temperature averages to consider climate change effects. All of these factors could have affected our catches, with lag effects and extreme values not necessarily reflecting temporal trends. Specifically, we used: the number of frost days (temperature below 0 °C), the precipitation sum and the average temperature of the year preceding the sampling (always week 16 of the previous year until week 15 of the sampling year), as well as the average temperature of the 5 years preceding the sampling.
To test for differences among the 3 years, we fitted generalized linear mixed models (function ‘glmmTMB’, R package glmmTMB; Brooks et al. 2017). The models included the year, habitat type, weather of the sampling week [mean weekly temperature (°C), rain (mm) and wind speed (m/s)] as fixed effects. The study site and week number were included as random effects, because we compared repeated measures from the same sites (Bolker et al. 2009). The weather variables were scaled to zero mean and unit variance prior to the analysis with the R function ‘scale’. As an additional term correcting for the fact that the number of species increases with the number of individuals caught, the log(Abundance) was included as fixed effect in the richness models. It was not possible to include climate variables of the previous year and 5-year averages into our models as they all were highly correlated with the factor year, reflecting continuous climate warming over the 32 year study period. We tested for model misspecification problems such as multicollinearity, over/underdispersion, zero-inflation residual, spatial and temporal autocorrelation with the R package DHARMa (Hartig 2020). If moderate or high collinearity of two fixed effects was detected, we excluded one of them. If overdispersion was detected, we included an individual level random effect and zero-inflation factor, respectively. Biomass data was analyzed as gauss distribution with log link, abundance and species richness data as poisson distribution with log link. Explicit formula per input variable (species richness, abundance, biomass) and trap types (pitfall traps, combi-traps, yellow + window traps) are given in Online Appendix S4.
In the end, we backtransformed the model’s estimates and pairwise compared the differences between the years with the R package emmeans (Lenth 2020).
To test for temporal changes in overall diversity in the study area, we additionally calculated gamma diversity (species diversity of the study area) for each year. To avoid any bias, sampling effort was equalized to the same trap types and numbers for each site (2 pitfall traps, 1 yellow bucket, 1 window trap). We used the R package iNEXT designed for rarefaction and extrapolation of species richness to correct for different numbers of sites sampled in the different years (Chao et al. 2014). We obtained diversity estimates for the Hill numbers q = 0, 1 and 2. Q = 0 equals species richness, q = 1 equals the exponential of Shannon entropy and q = 2 equals the inverse of Simpson diversity (Hill 1973). With increasing Hill number dominant species are more strongly weighted. Confidence intervals were calculated by bootstrapping (n = 200 bootstraps).
Results
Biomass, abundance and species richness
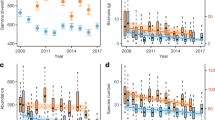
In total, 58,448 individuals from 1343 identified species were included in the analysis. The raw data are displayed in Fig. 2. By considering weather and site effects biomass was significantly higher in 2019 than 1997 (yellow + window and pitfall traps) and 1987 (only yellow + window traps) (Table 2; Fig. 3). Mean biomass per yellow + window trap in 2019 was twice as high (2688 mg) as in 1987 (1367 mg) and has increased by a factor of 1.8 since 1997 (1505 mg; Table 2; Fig. 3). According to the raw data, biomass in the yellow + window traps increased consistently in all habitat types for the three study years (Fig. 2). In the pitfall traps similar biomass was found in 1987 (2453 mg) compared to 2019 (2548 mg), but a 40% lower biomass in 1997 (1514 mg) compared to 2019. Differences in the pitfall traps were habitat-dependent, with a decrease in dry meadows and temporary grassland and an increase in wheat, whereas biomass in wet meadow was quite stable between years (Fig. 2).
Averaged total biomass, abundance, and species richness in four different habitat types for the years 1987, 1997 and 2019. The boxplots show the average numbers per trap week. Black lines show the median, 25th to 75th percentile of the data are within the boxes and the whiskers indicate 1.5*the interquartile range. Dots mark outliers
Standardized model estimates from the generalized linear mixed models testing for temporal trends in arthropod biomass, abundance, and species richness. The habitat dry meadow and the year 1987 serve as intercept, against which the model estimates are compared. Note that the values for temperature, precipitation, and wind speed for the study weeks were scaled prior to the analysis
Also abundance differed significantly between years with higher number of individuals caught in 2019 compared to 1997 (only pitfall traps) and 1987 (yellow + window and pitfall traps) as well as in 1997 compared to 1987 (yellow + window traps) (Table 2; Fig. 3). The average number of individuals in the yellow + window traps doubled between 1987 (69) and 2019 (138), while in 1997 (149) it was even slightly higher (Table 2). The abundance in the pitfall traps was 60% higher in 2019 (94) than in 1987 (60) and 1997 (56). Not all habitats showed the same pattern as the total (Fig. 2); we observed a general abundance increase in wheat, stable conditions in temporary grassland, an increase followed by a weaker decrease in wet meadows and a decrease followed by a stronger increase in dry meadows.
After controlling for differences in abundance, species richness was significantly higher in 2019 than 1997 (yellow + window traps) and 1987 (yellow + window and pitfall traps) as well as in 1997 compared to 1987 (pitfall traps) (Table 2). Raw data revealed that mean species richness in the yellow + window traps was 30% higher in 1997 than 1987 and 50% higher in 2019 (Fig. 2). Mean species richness increased consistently in all habitat types, except in dry meadows where it was lower in 1997 than the other 2 years. In the pitfall traps of 1997, the mean species richness was higher in all habitat types by 35% compared to 1987 and 20% in 2019 compared to 1997 except for the wet meadow, where it was a bit lower in 2019.
Species diversity of the study area (gamma diversity) was significantly lower in 1987 than in the other 2 years, reaching highest values in 2019 (Fig. 4, non-overlapping confidence intervals). This result was independent of the weighing of rare and common species (q-level). The differences between the years were more pronounced when common species were more strongly weighted. This shows that rare as well as common species increased in species richness, but the increase was strongest in common species.
Comparison of gamma diversity of the study area in the three study years. For each year, the data from two pitfall, one window and one yellow bucket trap per site (sampling unit) were combined over all study weeks. Solid lines are rarefied values, dashed lines extrapolated values. Species diversity was calculated in three ways with Hill numbers q = 0, 1 or 2. Q = 0 (left panel) equals species richness, q = 1 (middle panel) equals the exponential of Shannon entropy and q = 2 (right panel) equals the inverse of Simpson diversity. The higher q is, the heavier are frequent species weighted. Confidence intervals were calculated by bootstrapping (n = 200 bootstraps). Non-overlapping confidence intervals indicate significant differences
Data from the combi-traps over the 2 years 1997 and 2019 confirmed the patterns observed for the yellow + window traps (Fig. 2, Online Appendix S5). We found a similar strong increase in biomass from 1997 to 2019, similar abundances in both years and a higher species richness in 2019.
From 1997 to 2019, the increase in biomass in the flight traps was stronger than the increase in abundance. This suggests that some of the captured arthropods were heavier in 2019 than in 1997. However, we found no clear shift in the average body size of the community across the study years (Online Appendix S6).
Trends of individual taxa and trophic guilds
The most abundant taxa in the flight traps were the Coleoptera (besides the Diptera, of which only Syrphidae were evaluated), and both the Coleoptera and Araneae in the pitfall traps. Most insect groups, except for the Syrphidae and Neuroptera, Mecoptera and Raphidioptera, were abundant enough to infer individual trends. For the coleopteran families Carabidae and Staphylinidae, trends were evaluated separately. Trends for the Coleoptera (excl. Carabidae and Staphylinidae), Carabidae, Heteroptera and Aculeata were similar to the increasing trends of all taxa compiled (Fig. 5, Online Appendix S7). The Araneae increased in biomass and abundance but declined in species richness. For the Formicidae, no clear abundance or richness changes could be distinguished between the years. The taxon for which the trends deviate most from the overall trends were the Staphylinidae. They showed consistently lower biomass in 2019 than in 1997 and depending on the trap type different abundance and species richness trends.
Summary of the biomass, abundance and species richness trends derived from the raw data in total and separated by taxon and trophic guilds (Online Appendix S7). Trends for pitfall traps (PT) and yellow + window traps (Y + WT) over 3 years (1987, 1997, 2019). The arrows indicate the direction of the trends, for example an increase, decrease, no obvious changes among the study years, decrease followed by an increase or vice versa. Trends were not assessed for group-trap type combinations with a dash (–), as well as for the Syrphidae, Neuroptera, Raphidioptera and Mecoptera, as their abundances were too low. The trophic guild Other comprises detritivores, fungivores and trophobiotic species
Herbivores showed consistently increasing trends over the 3 years (Fig. 5, Online Appendix S7). Carnivores increased in biomass, abundance and species richness in the flight traps and showed no major changes in the pitfall traps. Omnivore species richness was clearly increasing, whereas biomass stayed stable and abundance trends were trap-dependent (the abundance in the pitfall traps was highest in 2019, whereas in the yellow + window traps it was highest in 1997).
Landscape change
Our analysis of landscape configuration since 1987 shows only minimal changes hinting towards loss of habitat quantity or quality. The forest cover remained stable (9.1 km2, 29.7% of total area). The area of agricultural land and gardens decreased by 0.3% (1985: 2058 ha, 2019: 2049 ha, 66.6% of total area) and was replaced mostly by buildings, which increased in area by 0.3% (1985: 31.7 ha, 2019: 39.2 ha) and a few new roads (1985: 282.3 km, 2019: 290.1 km).
Potential lag effects and climate change
Weather conditions led to a substantial increase in temperatures over the study period. Mean temperature in the preceding year to the sampling was 8.28 °C in 1987, 8.64 °C in 1997 and 10.39 °C in 2019. According to the Swiss weather archives (https://www.meteoswiss.admin.ch/home/climate/the-climate-of-switzerland/monats-und-jahresrueckblick/the-swiss-weather-archive.html), the period from spring 1986 to spring 1987 was with high precipitation, one of the coldest winters of the century and a cold, wet summer 1987. 1996 was an average year without much deviation from normal values, followed by the winter 1997, which had temperatures above normal. The summer of 2018 was then the hottest summer since measurements began and extremely dry. Also, the year 2019 was hotter than average, with only January and May clearly colder than average. Accordingly, the number of frost days decreased in our 3 sampling years (1986/87: 65 days, 1996/97: 39 days, 2018/19: 29 days) and so did the precipitation sum in the year preceding the sampling (1986/87: 1153 mm, 1996/97: 1000 mm, 2018/19: 699 mm). The comparison of the 5 year temperature average preceding the sampling (1982–1987: 8.32 °C, 1992–1997: 9.18 °C, 2014–2019: 9.72 °C) and the 5 year frost days average (1982–1987: 58.2 days, 1992–1997: 40.2 days, 2014–2019: 33.2 days) show less extreme differences but still a general warming of the climate (Online Appendix S11).
Discussion
We found higher arthropod biomass, abundance, and species richness per trap as well as higher overall regional species diversity in 2019 compared to the previous study years 1987 and 1997 in an intensively used agricultural landscape in Switzerland. Even after accounting for various effects (weather during sampling, habitat) in our statistical models, we often found the highest values in 2019. This correlates with the fact, that average temperatures also increased over each of the sampling years. Increases were largely consistent across taxonomic and trophic groups, which suggests that the respective environmental factors affected species independently of their taxonomic or trophic position and corroborates that our findings are reflective of changing diversity and abundance in Limpach valley region. However, increases were more prominent in flight traps than in pitfall traps, indicating that flight-active species were more responsive than the ground-dwellers.
Based on reports of strong insect declines in grasslands or nature reserves surrounded by agricultural land in Germany during the last decades (Seibold et al. 2019; Hallmann et al. 2017), we had expected to find similar results in the intensively used agricultural landscape of our study. In the following, we discuss why our findings might not be in line with the general expectation of negative biomass and richness trends.
First, in highly modified landscapes like the Limpach valley, major landscape and land-use changes that may have had strong negative impact on insect communities often happened in historic times (Habel et al. 2016; Ollerton et al. 2014). The wet Limpach valley was drained and turned into intensively cultivated land from 1900 to 1951 (Imhof 1988). The chosen baseline in our study is the year 1987. As this baseline is only around 30 years old, it might underestimate historical baseline situations (Didham 2020). Presumably, arthropod abundance and species declines happened decades before the 1980s—possibly leaving mainly generalist species surviving- and could since then be halted in the study area. Our results provide indications that source populations still exist in agricultural landscapes, at least in the one we studied, and can recolonize areas if improved e.g. by agri-enviromental schemes.
Second, measures to promote habitat quality were implemented in the Limpach valley over the study period. In the wetland reserve (Wengimoos, Western end of study area, Fig. 1) an agricultural field was restored to its natural state and habitat improvement measures were implemented in recent years (Friedli 2015). However, more far-reaching for the entire study area was probably the introduction of agri-environmental schemes in the 1990s: federal compensation payments are remunerated to farmers for dedicating parts of their land to measures beneficial for the environment or biodiversity (BAFU and BLW 2016). Currently, around 13% of the agricultural land are so called biodiversity promotion areas (BPAs) in the Swiss lowlands (BAFU and BLW 2016). They consist of different extensively used habitat types such as low intensity grasslands, traditional orchards, hedges, wild flower strips and fallow patches with restrictions on fertilization and mowing (Jeanneret et al. 2003). The respective proportion of BPAs in the Limpach valley is around 8% (https://geodienste.ch). Generally, areas under such an agri-environmental scheme have higher arthropod biodiversity and abundance (Aviron et al. 2009; Kleijn et al. 2006).
Third, pesticide use, nitrogen input and land-cover configuration remained comparable during the study period. Since 1987, pesticide use in Switzerland stayed within a similar range (de Baan et al. 2015). Nitrogen surplus and leaching decreased between 1990 and 2005 by around 20% (Decrem et al. 2007; Herzog et al. 2008) and remained stable since then (Bundesamt für Landwirtschaft BLW 2019). Detailed information about fertilizer and pesticide applications of the study sites were not available, but the Limpach valley is a representative agricultural area in the Swiss Central Plateau and the same crops are still cultivated as 32 years ago. The region is dominated since decades by agriculture and therefore landscape configuration changed only minimally indicating a minor loss of habitat quantity or quality. Few agricultural land and gardens were replaced by either buildings or new roads. However, most agricultural fields were merged and became larger but can still be considered as being small in international comparison. The relatively small-scale mosaic-like arrangement of agricultural and semi-natural habitats as well as bordering forests could have a positive effect on the arthropod community in the study area (Outhwaite et al. 2022).
Fourth, climate change has brought along an increase in average temperature coupled with a decrease of frost days and precipitation. All these factors were highly correlated with the year of sampling (Online Appendix S11), thus confounding factors for a comparative analysis. Nevertheless, the effects of climate change should also be considered as explanatory factors for the observed differences in species richness, abundance and biomass, although effects may even be species specific (Jackson et al. 2022). Beside the specific weather conditions in the study years (Online Appendix S10) also lag effects (weather conditions in preceding years, frost days in previous winter) could have affected our catches. We could not analyze the role of lag effects statistically as these factors highly correlated with the factor year. However, we found an increase in average temperature of the previous year, an increase of the preceding 5 year’s average temperature and a decrease of number of frost days over the study period. These values thus reflect the long-term climate warming.
The influence of weather conditions is a challenge in estimating arthropod abundances. Weather conditions during sampling influence the catching probability of arthropods. Higher temperatures and relatively dry weather for instance lead to higher activity of arthropods, which enhances the catching probability (Yi et al. 2012). We tried to account for the influence of weather conditions by including them in the mixed effects models and by adapting the sampling scheme (see “Methods”). However, essentially all quantitative insect sampling methods suffer from this effect (Didham et al. 2020), which means that also in studies finding insect declines this bias is present.
The influence of climate warming is very complex, there are winners and losers among the insects and it is difficult to say, if climate warming results in a net gain or loss of insect biomass, abundance and species richness. Warmer temperatures are associated with higher overwintering survival, more generations and the immigration of warm adapted species (Vittoz et al. 2013), which might explain the higher species richness in recent years. On the other hand, warmer temperatures, fewer frost days, less rain and altered vegetation periods can also lead to altered prey-predator interactions (Barton and Ives 2014), asynchronous development or distribution range shifts (Robinet and Roques 2010), which might have a negative impact on insect communities. Especially in the tropics, climate change is supposed to be a main driver of insect declines (Wagner 2020). In temperate regions however, many forests have become much more suitable for a number of species due to climate change (Pureswaran et al. 2018). As our study area is surrounded by forests, this might also partly explain the higher number of species observed in the last year of observation. In addition, the gamma diversity analysis indicates, that the higher species diversity in 2019 might be due to increases in more common species.
In general, in studies looking at insect trends, great variation can be found even in geographically close sites (van Klink et al. 2020) or between different habitats in the same landscape. For example, in Britain, moth biomass declined in woodland, grassland and urban environments, but not in—likely already depleted—arable land (Macgregor et al. 2019). Contrastingly, carabid beetle abundance declined in moorland and pastures, but increased in woodland over a period of 15 years (Brooks et al. 2012). The reasons for differences in trends between regions and habitats are still largely unknown and thus every study can contribute to the understanding of the bigger picture.
When evaluating long-term insect population trends, one has to consider many challenges and pitfalls involved (Didham et al. 2020). We are well aware of the limited inference from the data presented in our study from a single region and a snapshot comparison of only three timepoints. Estimates of population change can be sensitive to selection bias effects in the choice of the time-points, much as described for false baseline effects above. Population densities of arthropod communities and therefore their biomass is known to fluctuate substantially from year to year. Unfortunately, at the time of writing only few comparative long-term datasets on arthropod communities exist for Switzerland that allow to estimate the natural range of such fluctuations. To cite a few international ones, moth biomass in Britain varied twofold within a decade (Macgregor et al. 2019), whereas carabid beetle biomass varied up to five fold within 10 years (Homburg et al. 2019). Biomass fluctuations of whole arthropod communities, as assessed in our study, are less pronounced than those of single taxonomic units. In the study of Seibold et al. (2019) the whole community varied 2- to 3-fold per decade. In our study, biomass increased 1.5-fold in the pitfall traps and 3-fold in the yellow + window traps as well as in the combi-traps. If we assume a scenario of a substantial abundance and biomass decline over the 32 years, our findings of substantial increases in the flight traps likely outreach the range of natural fluctuations. A measure of yearly fluctuations in species richness gives us the study of Obrist and Duelli (2010). From 2000 to 2007, arthropod species richness was monitored at 45 locations in Switzerland. Yearly fluctuations averaged 6%, with the highest difference reaching 20% between any of the 8 years. Warmer years yielded more species.
To estimate the potential bias of our three sampling years being exceptional, we evaluated these years in comparison to earlier and later years based on other studies. The butterfly abundance data from the long-term Swiss biodiversity monitoring (http://www.biodiversitymonitoring.ch/en/home.html) clearly shows that 2019 was a year with average abundances, even though it was a rather hot year and preceded by a very hot year with a peak in abundances (Online Appendix S8). Interesting is, that also butterfly abundance increased in the Swiss Central Plateau between 2003 and 2019. Similarly, the abundance of water insects increased between 2010 and 2019 (Online Appendix S8). Primarily generalist butterfly species with low habitat specialization have increased, as well as species that respond positively to the establishment of biodiversity promotion areas (BPAs) in agricultural areas. Certain warm adapted species have also become more common (Widmer et al. 2021), which could also be the case in our study. In order to estimate the representativeness of the years 1987 and 1997, we compared mean abundance and species richness per trap with data collected in other, similar studies conducted by the Swiss Federal Research Institute WSL between 1980 and 2000 (Online Appendix S9). The years 1987 and 1997 did not stand out as years with exceptionally low or high insect abundances, which indicates our results to represent average years. In terms of weather conditions, 1987 was colder than average whereas 1997 was a more or less normal year.
In our study single taxonomic groups and trophic guilds mostly followed the overall increasing trends. Carabid beetles (Coleoptera: Carabidae) as well as most other beetle families showed mainly higher biomass, abundance, and diversity in 2019 than in the previous study years. In other countries either stable abundance and biomass (Homburg et al. 2019) or declining trends were found for these groups (Brooks et al. 2012; Ewald et al. 2015; Hallmann et al. 2020; Harris et al. 2019). Only the rove beetles (Coleoptera: Staphylinidae) showed somewhat diverging and often declining abundance and richness patterns in our study. However, other comparative long-term studies are lacking and prevents any comparisons. In another Swiss agricultural landscape, ground-living spiders (Araneae) were found to decline in abundance by 8% between 1994 and 2004 (Blandenier et al. 2014), but in our case spider abundance increased while diversity decreased. This is in line with the finding that biodiversity promotion areas do not seem to enhance spider diversity (Aviron et al. 2009). Despite reports of declining local bee diversity in various countries like Britain, Netherlands or Spain (Bartomeus et al. 2018; Biesmeijer et al. 2006), we found a consistently higher species richness for these important pollinators (Hymenoptera: Aculeata). As a last taxon, for which trends could be inferred, the true bugs (Hemiptera: Heteroptera) seem to be generally less affected by declines, neither in our study nor in Dutch nature reserves (Hallmann et al. 2020) or in cereal fields in England (Ewald et al. 2015).
To draw a more comprehensive picture, further repetitions of former arthropod surveys and studies would be needed. The only standardized monitoring of arthropods across different habitats of Switzerland is the Swiss biodiversity monitoring (http://www.biodiversitymonitoring.ch/en/home.html), which is limited to butterflies and aquatic insects (Ephemeroptera, Plecoptera, Trichoptera). It would be most important to complement the Swiss biodiversity monitoring with additional, functionally different insect taxa, including their abundances, to elucidate possible causes of declines or increases.
Conclusions
We consider (a) the ecological restoration and enlargement of the wetland reserve, (b) the integration of biodiversity promotion areas into the agricultural land since 1993, (c) a relatively stable landscape configuration and habitat composition, (d) a stable to declining use of pesticides and fertilizers, and (e) the warming climate as the main drivers of the apparent increases in arthropod biomass and diversity in the Limpach valley between 1987 and 2019. The influence of weather and climate warming are context-dependent and cannot be assessed conclusively with our existing data. However, climate change should similarly have affected arthropod populations in other studies, in which massive insect declines were observed. Since we only treat one focus area in our study, we abstain from generalizing our results. We also emphasize the shifting baseline syndrome: positive trends are easier to achieve in systems that have been impoverished in historic times already. To fill the gap between contrasting changes in arthropod biodiversity, we encourage further long-term investigations on arthropod dynamics in regions with different management history and types, to reveal the drivers of the spatial differences in temporal patterns of arthropod abundance, biomass and diversity.
Data availability
Code availability
Code will be uploaded on https://doi.org/10.16904/envidat.163 as soon as the paper is accepted for publication.
References
Aviron S, Nitsch H, Jeanneret P, Buholzer S, Luka H, Pfiffner L, Pozzi S, Schüpbach B, Walter T, Herzog F (2009) Ecological cross compliance promotes farmland biodiversity in Switzerland. Front Ecol Environ 7:247–252. https://doi.org/10.1890/070197
BAFU, BLW (2016) Umweltziele Landwirtschaft. Statusbericht 2016. Bundesamt für Umwelt. Bern (Umwelt-Wissen 1633, 1633)
Bartomeus I, Stavert JR, Ward D, Aguado O (2018) Historical collections as a tool for assessing the global pollination crisis. Philos Trans R Soc B 374:20170389. https://doi.org/10.1098/rstb.2017.0389
Barton BT, Ives AR (2014) Direct and indirect effects of warming on aphids, their predators, and ant mutualists. Ecology 95:1479–1484. https://doi.org/10.1890/13-1977.1
Belovsky GE, Slade JB (2000) Insect herbivory accelerates nutrient cycling and increases plant production. PNAS 97:14412–14417. https://doi.org/10.1073/pnas.250483797
Bianchi FJJA, Booij CJH, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc Biol Sci 273:1715–1727. https://doi.org/10.1098/rspb.2006.3530
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354. https://doi.org/10.1126/science.1127863
Blandenier G, Bruggisser OT, Bersier L-F (2014) Do spiders respond to global change? A study on the phenology of ballooning spiders in Switzerland. Écoscience 21:79–95. https://doi.org/10.2980/21-1-3636
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol (Amst) 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Bowler DE, Heldbjerg H, Fox AD, Jong M, de, Böhning-Gaese K (2019) Long-term declines of european insectivorous bird populations and potential causes. Conserv Biol 33:1120–1130. https://doi.org/10.1111/cobi.13307
Brooks DR, Bater JE, Clark SJ, Monteith DT, Andrews C, Corbett SJ, Beaumont DA, Chapman JW (2012) Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. J Appl Ecol 49:1009–1019. https://doi.org/10.1111/j.1365-2664.2012.02194.x
Brooks M, Kristensen K, Benthem K, Magnusson A, Berg C, Nielsen A, Skaug H, Mächler M, Bolker B (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized Linear mixed modeling. R J 9:378. https://doi.org/10.32614/RJ-2017-066
Bundesamt für Landwirtschaft BLW (2019) Agrarbericht 2019. https://www.agrarbericht.ch. Accessed 24 May 2020
Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kwak ML, Mammola S, Ari Noriega J, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Stork NE, Suhling F, Vorster C, Samways MJ (2020) Scientists’ warning to humanity on insect extinctions. Biol Conserv 242:108426
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Crossley MS, Meier AR, Baldwin EM, Berry LL, Crenshaw LC, Hartman GL, Lagos-Kutz D, Nichols DH, Patel K, Varriano S, Snyder WE, Moran MD (2020) No net insect abundance and diversity declines across US Long Term Ecological Research sites. Nat Ecol Evol 4:1368–1376. https://doi.org/10.1038/s41559-020-1269-4
de Baan L, Spycher S, Daniel O (2015) Einsatz von Pflanzenschutzmitteln in der Schweiz von 2009 bis 2012. Agrarforschung Schweiz 6:48–55
Decrem M, Spiess E, Richner W, Herzog F (2007) Impact of swiss agricultural policies on nitrate leaching from arable land. Agron Sustain Dev 27:243–253. https://doi.org/10.1051/agro:2007012
Didham RK, Basset Y, Collins CM, Leather SR, Littlewood NA, Menz MHM, Müller J, Packer L, Saunders ME, Schönrogge K, Stewart AJA, Yanoviak SP, Hassall C (2020) Interpreting insect declines: seven challenges and a way forward. Insect Conserv Divers 13:103–114. https://doi.org/10.1111/icad.12408
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B (2014) Defaunation in the Anthropocene. Science 345:401–406
Duelli P, Obrist MK (2003) Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl Ecol 4:129–138. https://doi.org/10.1078/1439-1791-00140
Duelli P, Obrist MK, Schmatz DR (1999) Biodiversity evaluation in agricultural landscapes: above-ground insects. Agric Ecosyst Environ 74:33–64. https://doi.org/10.1016/S0167-8809(99)00029-8
Ewald JA, Wheatley CJ, Aebischer NJ, Moreby SJ, Duffield SJ, Crick HQP, Morecroft MB (2015) Influences of extreme weather, climate and pesticide use on invertebrates in cereal fields over 42 years. Glob Change Biol 21:3931–3950. https://doi.org/10.1111/gcb.13026
Friedli D(2015) Das Naturschutzgebiet Wengimoos. https://www.ala-schweiz.ch/images/reservate/_pdf/2015chronikwengimoos.pdf. Accessed 23 January 2020
Gallai N, Salles J-M, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821. https://doi.org/10.1016/j.ecolecon.2008.06.014
Gossner MM, Simons NK, Achtziger R, Blick T, Dorow WHO, Dziock F, Köhler F, Rabitsch W, Weisser WW (2015) A summary of eight traits of Coleoptera, Hemiptera, Orthoptera and Araneae, occurring in grasslands in Germany. Sci Data 2:150013. https://doi.org/10.1038/sdata.2015.13
Gygax R (1999) Methodenoptimierung zur Schätzung der lokalen Biodiversität von Arthropoden. Diplomarbeit. https://doi.org/10.16904/envidat.163
Habel JC, Segerer A, Ulrich W, Torchyk O, Weisser WW, Schmitt T (2016) Butterfly community shifts over two centuries. Conserv Biol 30:754–762. https://doi.org/10.1111/cobi.12656
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H (2017) More than 75% decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. https://doi.org/10.1371/journal.pone.0185809
Hallmann CA, Zeegers T, Klink R, Vermeulen R, Wielink P, Spijkers H, Deijk J, Steenis W, Jongejans E (2020) Declining abundance of beetles, moths and caddisflies in the Netherlands. Insect Conserv Divers 13:127–139. https://doi.org/10.1111/icad.12377
Harris JE, Rodenhouse NL, Holmes RT (2019) Decline in beetle abundance and diversity in an intact temperate forest linked to climate warming. Biol Conserv 240:108219. https://doi.org/10.1016/j.biocon.2019.108219
Hartig F (2020) DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. https://CRAN.R-project.org/package=DHARMa
Herzog F, Prasuhn V, Spiess E, Richner W (2008) Environmental cross-compliance mitigates nitrogen and phosphorus pollution from swiss agriculture. Environ Sci Policy 11:655–668. https://doi.org/10.1016/j.envsci.2008.06.003
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. https://doi.org/10.2307/1934352
Homburg K, Drees C, Boutaud E, Nolte D, Schuett W, Zumstein P, Ruschkowski E, Assmann T (2019) Where have all the beetles gone? Long-term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conserv Divers 67:268–277. https://doi.org/10.1111/icad.12348
Imhof T(1988) Wengimoos bei Büren. OB Beiheft 7, 27–31. https://www.ala-schweiz.ch/images/reservate/_pdf/Beiheft_1988_85_27_Wengimoos.pdf. Accessed 24 May 2020
Jackson HM, Johnson SA, Morandin LA, Richardson LL, Guzman LM, M’Gonigle LK (2022) Climate change winners and losers among North American bumblebees. Biol Lett 18:20210551. https://doi.org/10.1098/rsbl.2021.0551
Jeanneret P, Schüpbach B, Pfiffner L, Herzog F, Walter T (2003) The swiss agri-environmental programme and its effects on selected biodiversity indicators. J Nat Conserv 11:213–220. https://doi.org/10.1078/1617-1381-00049
Jenni L, Graf R (2018) Rückgang der Insektenfresser. In: Knaus P, Antoniazza S, Wechsler S, Guélat J, Kéry M, Strebel N, Sattler T (eds) Schweizer Brutvogelatlas 2013–2016: Verbreitung und Bestandsentwicklung der Vögel in der Schweiz und im Fürstentum Liechtenstein. Schweizerische Vogelwarte, Sempach, Switzerland, pp 304–305
Kleijn D, Baquero RA, Clough Y, Díaz M, de Esteban J, Fernández F, Gabriel D, Herzog F, Holzschuh A, Jöhl R, Knop E, Kruess A, Marshall EJP, Steffan-Dewenter I, Tscharntke T, Verhulst J, West TM, Yela JL (2006) Mixed biodiversity benefits of agri-environment schemes in five european countries. Ecol Lett 9:243–254. https://doi.org/10.1111/j.1461-0248.2005.00869.x
Lamarre GPA, Pardikes NA, Segar S, Hackforth CN, Laguerre M, Vincent B, Lopez Y, Perez F, Bobadilla R, Silva JAR, Basset Y (2022) More winners than losers over 12 years of monitoring tiger moths (Erebidae: Arctiinae) on Barro Colorado Island, Panama. Biol Lett 18:20210519. https://doi.org/10.1098/rsbl.2021.0519
Lenth R(2020) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.4. R package version 0.3.3.0. https://CRAN.R-project.org/package=emmeans
Macgregor CJ, Williams JH, Bell JR, Thomas CD (2019) Moth biomass increases and decreases over 50 years in Britain. Nat Ecol Evol 3:1645–1649. https://doi.org/10.1038/s41559-019-1028-6
Maes D, van Dyck H (2001) Butterfly diversity loss in Flanders (north Belgium): Europe’s worst case scenario? Biol Conserv 99:263–276. https://doi.org/10.1016/S0006-3207(00)00182-8
Obrist MK, Duelli P (2010) Rapid biodiversity assessment of arthropods for monitoring average local species richness and related ecosystem services. Biodivers Conserv 19:2201–2220. https://doi.org/10.1007/s10531-010-9832-y
Ollerton J, Erenler H, Edwards M, Crockett R (2014) Pollinator declines. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346:1360–1362. https://doi.org/10.1126/science.1257259
Outhwaite CL, McCann P, Newbold T (2022) Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 605:97–102. https://doi.org/10.1038/s41586-022-04644-x
Pureswaran DS, Roques A, Battisti A (2018) Forest insects and climate change. Curr Forest Rep 4:35–50. https://doi.org/10.1007/s40725-018-0075-6
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Robinet C, Roques A (2010) Direct impacts of recent climate warming on insect populations. Integr Zool 5:132–142. https://doi.org/10.1111/j.1749-4877.2010.00196.x
Schuch S, Wesche K, Schaefer M (2012) Long-term decline in the abundance of leafhoppers and planthoppers (Auchenorrhyncha) in central european protected dry grasslands. Biol Conserv 149:75–83. https://doi.org/10.1016/j.biocon.2012.02.006
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze E-D, Vogt J, Wöllauer S, Weisser WW (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674. https://doi.org/10.1038/s41586-019-1684-3
Sohlström EH, Marian L, Barnes AD, Haneda NF, Scheu S, Rall BC, Brose U, Jochum M (2018) Applying generalized allometric regressions to predict live body mass of tropical and temperate arthropods. Ecol Evol 8:12737–12749. https://doi.org/10.1002/ece3.4702
Soroye P, Newbold T, Kerr J (2020) Climate change contributes to widespread declines among bumble bees across continents. Science 367:685–688. https://doi.org/10.1126/science.aax8591
Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Fox R, Clarke RT, Lawton JH (2004) Comparative losses of british butterflies, birds, and plants and the global extinction crisis. Science 303:1879–1881. https://doi.org/10.1126/science.1095046
van Klink R, Bowler DE, Gongalsky K, Swengel AB, Gentile A, Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368:417–420. https://doi.org/10.1126/science.aax9931
van Klink R, Bowler DE, Gongalsky KB, Chase JM (2022) Long-term abundance trends of insect taxa are only weakly correlated. Biol Lett 18:20210554. https://doi.org/10.1098/rsbl.2021.0554
Vaughan N (1997) The diets of british bats (Chiroptera). Mammal Rev 27:77–94. https://doi.org/10.1111/j.1365-2907.1997.tb00373.x
Vittoz P, Cherix D, Gonseth Y, Lubini V, Maggini R, Zbinden N, Zumbach S (2013) Climate change impacts on biodiversity in Switzerland: a review. J Nat Conserv 21:154–162. https://doi.org/10.1016/j.jnc.2012.12.002
Wagner DL (2020) Insect declines in the Anthropocene. Annu Rev Entomol 65:457–480. https://doi.org/10.1146/annurev-ento-011019-025151
Wagner DL, Fox R, Salcido DM, Dyer LA (2021) A window to the world of global insect declines: moth biodiversity trends are complex and heterogeneous. PNAS. https://doi.org/10.1073/pnas.2002549117
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: death by a thousand cuts. PNAS. https://doi.org/10.1073/pnas.2023989118
Widmer I, Mühlethaler R, Baur B, Gonseth Y, Guntern J, Klaus G et al (2021) Insektenvielfalt in der Schweiz: Bedeutung, Trends, Handlungsoptionen
Yi Z, Jinchao F, Dayuan X, Weiguo S, Axmacher JC (2012) A comparison of terrestrial arthropod sampling methods. J Resour Ecol 3:174–182. https://doi.org/10.5814/j.issn.1674-764x.2012.02.010
Acknowledgements
We thank H. Luka, Y. Chittaro, X. Heer, H. Martz, C. Germann, M. Herrmann and R. Bärfuss for the arthropod species identification and D. Schneider for her technical support. We thank Regula Gygax† for collecting the 1997 data. We also thank the farmers and the Office for Agriculture and Nature of the canton Bern for the permission to set up traps on their land and the Federal Office for the Environment (FOEN) for providing the data from the BDM (Swiss biodiversity monitoring). The study was financed by a WSL-internal research grant.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL. The study was financed by an internal research grant from the Swiss Federal Institute for Forest, Snow and Landscape Research.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology: MO, KB, JF, PD; Formal analysis and investigation: JF, MO, MG; Writing—original draft preparation: JF; Writing—review and editing: MO, KB, MG, PD; Funding: WSL-internal.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fürst, J., Bollmann, K., Gossner, M.M. et al. Increased arthropod biomass, abundance and species richness in an agricultural landscape after 32 years. J Insect Conserv 27, 219–232 (2023). https://doi.org/10.1007/s10841-022-00445-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00445-9