Abstract
Mulching, cutting of the vegetation without plant biomass removal, is a common alternative to mowing. The aim of our study was to find out if the mulching of forest meadows at different time points affects cavity-nesting bees and wasps. We exposed trap nests for cavity-nesting bees and wasps at 24 forest meadows in south-western Germany over 2 years and applied four experimental mulching treatments with six replicates: (i) mulching in June, (ii) mulching in September, (iii) mulching in June and September, and (iv) no mulching as control. Nests were collected throughout the growing period. The insects were sorted and analyzed according to functional groups. Mulching in June and September reduced the nest number of all cavity-nesting insects in the second but not in the first year. The separation of insects into three functional groups (bees, herbivore-hunting wasps and carnivore-hunting wasps) showed that the number of herbivore-hunting wasp nests was reduced by mulching in September in both years and by mulching in June and September in the second year. Specifically, aphid-hunting wasps were influenced by mulching in September or mulching twice in the second year. Aphid-hunting wasps likely find their larval food in the vegetation of the forest meadows, while the other studied groups likely find their main larval food in the surrounding forests and are therefore not negatively affected.
Implications for insect conservation
For maintaining the reproductive success of cavity-nesting wasps that hunt for aphids, we recommend mulching once in June rather than mulching in September or twice a year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biodiversity of meadows is influenced by several factors, for example the timing and frequency of cutting, the amount and type of fertilization and the geographic location (Gilhaus et al. 2017; Rannap et al. 2017; Schuch et al. 2012; Sullivan et al. 2018). Many insects depend on species-rich grasslands (Ebeling et al. 2018; Knops et al. 1999; Schuch et al. 2012). A special type of meadows are forest meadows, characterized by small grassland patches (around 1 ha) surrounded by forests. Forest meadows were e.g., established to increase the food supply for roe deer (Capreolus capreolus) and red deer (Cervus elaphus), for hunting and to protect tree seedlings and saplings in forests from harmful browsing (Erlacher and Völk 2003; Petrak 2003). The focus on game animal control and the exclusion of livestock is reflected by their alternative name “game meadow”. Towards the end of nineteenth century, these meadows increased in number and most still persist as managed forest meadows (Völk 1999). Due to the uniformity of the forests surrounding these meadows they provide a suitable study system. Compared to other grasslands, forest meadows are infrequently studied in the scientific literature (but see Aboling 2003; Petrak 2003; Petrak et al. 2015; Tomić et al. 2010). Only Aboling (2003) mentioned game meadows as interesting for game animals and vegetation studies, Petrak (2003) and (2015) gave practical instructions how to provide food for game animals and integrate nature conservation in game meadow management and a study by Tomić et al. (2010) described the plant communities on game meadows in Serbia.
Insects are currently declining in many parts of the world (Hallmann et al. 2021; Sánchez‐Bayo and Wyckhuys 2021; Seibold et al. 2019; Wagner et al. 2021). The main explanations for insect decline are the loss of habitat through increasing urbanization, climate change, landscape homogenization, high use of pesticides and fertilization and intensive farming methods for example frequent mowing (IPBES 2019; Dicks et al. 2021; Mupepele et al. 2019; Potts et al. 2010).
Cavity-nesting bees and wasps comprise around 5–10% of all solitary bee and wasp species in different parts of the globe (calculation based on species numbers given in Krombein (1967) and Tscharntke et al. (1998). However, they were shown to be sensitive to habitat changes (Staab et al. 2018; Tscharntke et al. 1998). This is backed up by many studies showing the negative influences of habitat change and land-use intensification for cavity-nesting bee and wasp communities (e.g. Steffan-Dewenter 2002; Tylianakis et al. 2006). Steffan-Dewenter (2002) showed, for example, that the structure and composition of the landscape adjacent to the exposed trap nests influenced the diversity of cavity-nesting bees and wasps, while Tylianakis et al. (2006) showed changes in the cavity-nesting bee and wasp communities along a gradient of land-use intensification involving rice fields, grasslands, agroforestry, and forest habitats. Bees and wasps are involved in different ecosystem functions like herbivore control (Harris 1994) and pollination (Klein et al. 2007). Evaluating the effects of forest meadow management on the number of established bee and wasp nests is crucial for guiding future insect conservation in forest meadows and beyond.
Cavity-nesting bees and wasps can be separated, based on their natural history, into three major functional groups. The three major functional groups are: (1) bees provisioning their larvae with pollen provided by flowers; (2) herbivore-hunting wasps provisioning their larvae for example with moth and beetle larvae or aphids which they collect from vegetation; (3) carnivore-hunting wasps, in our study mainly represented by spider-hunting wasps, collecting spiders in the surroundings of their nests (Westrich 1996). By grouping cavity-nesting bees and wasps into these three functional groups, we can test the individual response of these major functional groups to the mulching management.
The aim of the study is to find out if the mulching of forest meadows at different times affects cavity-nesting bee and wasp nest numbers in trap nests and if the responses differ between major functional groups, to provide management recommendations. Therefore, we have the following three hypotheses:
Meadow mulching directly reduces short-term flower availability (Cizek et al. 2012). We therefore expect mulching twice a year to decrease the number of cavity-nesting bee nests (hypotheses 1). Mulching also directly reduces herbivorous insect larvae attached to meadow plants (Humbert et al. 2010), which are suitable food resources for herbivore-hunting wasps (Westrich 1996). Especially in August and September insect larvae and aphid abundance is high (Chung et al. 1980; Holmes et al. 1979). Hence, we assume mulching twice a year and mulching in September will reduce the number of herbivore-hunting wasp nests compared to the control (hypotheses 2). In our study, carnivore-hunting wasps depended mainly on spiders as a food resource. Spider abundance increased with the succession of grasslands to forest (Brauckmann 2013) and spider-hunting wasps are known to find their prey predominantly in forests (Rypstra et al. 2007). Therefore, if these wasps hunt in the surrounding forest and not on the meadows, we expect there will be no effect of mulching on the number of carnivore-hunting wasp nests (hypotheses 3).
Materials and methods
Study region
The 10,000 ha Black Forest National Park is located in Baden-Württemberg and was founded in January 2014 (Förschler 2015). The national park is divided into three zones: core, transition and management zone (Förschler 2015) and the examined 24 forest meadows were distributed over all three zones located in the southern part of the Black Forest National Park (Fig. 1A). The national park belongs to the regions with the highest annual precipitation rates between 1400 and 2200 mm of Germany (DWD 2019). The annual mean air temperature ranges between 5 and 7 °C (Landesanstalt für Umwelt Baden-Württemberg [LUBW] 2006) and days with a maximum temperature of > 25 °C occurs 5–15 times annually (LUBW 2006) resulting in a short growing season.
The 24 examined forest meadows were located between 570 and 1005 m.a.s.l. and are predominantly surrounded by coniferous forests (dominated by spruce, Picea abies), which were managed for timber production prior to 2014 (Fig. 1B). The 24 forest meadows were created from 10 to more than 150 years ago for hay making, hunting purposes and for use as tree nurseries. In the last 5–30 years, forest meadows in the study region were founded and managed mainly for hunting. Since 2014 the meadows were managed by mulching in June and no fertilization. Management information before 2014 was not available for each meadow in detail (Tschöpe et al. 2017, 2018). Soil nutrient availability on all 24 examined forest meadows can be considered as poor (Buse et al. 2018).
The selection of the forest meadows was based on the following criteria: (1) Even distribution across the area of the national park (measured in QGIS using distance measurement, the mean minimum distance was 1112 m (± 401 m), the mean maximum distance was 10,617 m (± 2529 m), (2) uniform slope of the meadows with a preference for flat meadows (cut-off slope value was 10°), (3) surrounded by forest.
To test the effect of mulching time and no mulching (control) on cavity-nesting bees and wasps, we established four different treatments in 2017 and 2018. In both study years the same 24 meadows were used and the same treatment was applied for both years on each meadow. We chose this design as management effects are not always detected in the same year but rather in consecutive years (Eckerter et al. 2021). Mulching was conducted as cutting and shredding the vegetation and its direct deposition on the meadow (Schreiber et al. 2013). This procedure commonly applied by local authorities is considered cost efficient management. The 24 meadows were divided into four management groups (i) six meadows with mulching in June, (ii) six meadows with mulching in September, (iii) six meadows with mulching twice, once in June and once in September and (iv) six meadows without mulching treatment (control). The mulching was conducted at beginning of the month mentioned above at all sites within 5 days, during dry weather between 10 am and 6 pm. A Fendt mulching machine (Marktoberdorf, Germany) was used. The machine cut the grass above 5 cm, shredded the grass, and then distributed it evenly over the forest meadow.
Cavity-nesting bee and wasp survey
We surveyed the cavity-nesting bee and wasp community using trap nests in 24 different forest meadows. In each forest meadow, we installed four posts equipped with two trap nests each. The posts were placed along the forest edge and orientated towards southeast. Following Tscharntke et al. (1998) the trap nests consisted of 20 cm long PVC tubes, each with a diameter of 13 cm. Each of these tubes contained about 180 internodes of common reed grass, Phragmites communis. In April, July and October 2017 and 2018, all occupied reed internodes (reed internodes in which a bee or wasp made provisioned a nest) were collected from the trap nests and stored in test tubes closed with cotton until March at 4 °C, to simulate hibernation. The walk-in freezer used for hibernation had a dehumidifier to prevent mold. During each winter, the reed internodes were opened and the functional bee and wasp groups and their larval food resource were determined based on nest structure and content (Tscharntke et al. 1998). The reared imagos were stored in the insect collection of the Black Forest National Park. Species level identification was conducted for a subset of individuals but not further used for analyses.
Statistical analyses
For each meadow and sampling year, we pooled the data from the eight trap nests of each meadow and the three sampling dates to calculate the total number of nests, and the number of nests, separated into the three functional groups: bees, herbivore-hunting wasps and carnivore-hunting wasps on each meadow and separated into the nest number of the aphid-hunting wasps, larvae-hunting-wasps, barklice-hunting wasps, spider-hunting wasps, and fly-hunting wasps.
Generalized linear mixed models [GLMMs, package ‘lme4’ (Bates et al. 2018)] were used to test the effect of the time of mulching on the total number of nests and the number of nests of the different functional groups separately and for each year separately (Eq. 1). We evaluated the model assumptions using the package ‘DHARMa’ (Hartig 2017) and used an observation level random effect (olre), to account for overdispersion.
The statistical analyses were performed in R, version 3.6.2 (R Core Team 2019).
Results
We collected 3241 nests in 2017, and 4153 nests in 2018. In 2017, bees constructed 149 nests, herbivore-hunting wasps 1968 nests and carnivore-hunting wasps 1124 nests. In 2018, bees constructed 778 nests, herbivore-hunting wasps 2235 nests, carnivore-hunting wasps 1140 nests.
In 2017 the herbivore-hunting wasp nests comprised 84% of aphid-hunting wasp nests and in 2018, 76% of the herbivore-hunting wasps were aphid hunters. The other nests from herbivore-hunting wasps were built from caterpillar-, beetle larvae- and barklice-hunting wasps. The carnivore-hunting wasp nests were mainly spider-hunting wasp nests and a few fly-hunting wasp nests (< 2% in 2017 and < 1% in 2018) (Table 1).
While the nest numbers of the herbivore and spider-hunting wasps stayed similar in both years, the bee nest number was around 5 times higher in 2018 compared to 2017. Also, the beetle-larvae hunting wasp nests and the barklice-hunting wasp nest numbers were more than 3 times higher in 2017 than in 2018. We did not identify all imagos at the species level but for an approximation of the species found in the observed community see Supplementary Table 1 (for a list of flowering plant species see Supplementary Table 2).
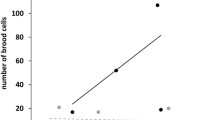
First, we examined the total number of nests in both years. In 2017, the total number of nests was not affected by mulching. In 2018, the total number of nests was reduced in meadows which were mulched twice, in June and in September, (Estimate = − 0.64, std. error = 0.32, z-value = − 2.04, p = 0.04) (Fig. 2). All other mulching times had no effect different from the control.
Second, we analyzed the three functional groups: (1) bees, (2) herbivore-hunting wasps and (3) carnivore-hunting wasps. In both years, the number of nests of herbivore-hunting wasps declined in the September-mulched meadows (2017; Estimate = − 0.70, std. error = 0.35, z-value = − 2.01, p = 0.04 | 2018; Estimate = − 0.84, std. error = 0.38, z-value = − 2.19, p = 0.03). In 2018, (second sampling year), the herbivore-hunting wasp nest number was also reduced by mulching twice (2018; Estimate = − 1.18, std. error = 0.38, z-value = − 3.07, p = 0.00) compared to the control (Fig. 3). The nest numbers of the bees and spider-hunting wasps were not affected by our treatments.
Effect of mulching time on the number of nests of the three functional groups (bees, herbivore-hunting wasps, carnivore-hunting wasps) in 2017 and 2018. Each mulching treatment was compared to the control. Significant differences between mulching times are indicated by different letters for each functional group
As the herbivore-hunting wasp nests in our study mainly consisted of aphid-hunting wasp nests and carnivore-hunting wasp nests made mainly out of spider-hunting wasp nests, we analyzed, in a third step, these two groups in more detail: They were separated into aphid-hunting wasps, larvae-hunting-wasps and spider-hunting wasps. We could not analyze the barklice- and fly-hunting wasp nests and we had to sum up the caterpillar- and beetle larvae-hunting wasp nests to larvae-hunting wasp nests because we had too few nests of that type. The result was, that only aphid-hunting wasps reduced their nest numbers in the second sampling year through mulching in September and mulching twice (2018 mulching in September; Estimate = − 1.08, std. error = 0.54, z-value = − 1.99, p = 0.05 | 2018 mulching twice; Estimate = − 1.44, str. error = 0.54, z-value = − 2.66, p = 0.01). Spider-hunting wasps and larvae-hunting wasps were not affected by our treatment.
Discussion
We confirmed the expected negative effect of mulching on aphid-hunting wasps and showed that mulching in September or twice a year is responsible for this pattern. However, bees, larvae-hunting wasps and spider-hunting wasps were not affected by mulching. Therefore, the results show that even highly mobile, flying insects nesting above the meadow vegetation are affected by the removal and mulching of living plant biomass, but depending on the functional group and food resource, species respond differently. Nevertheless, this study on three distinct functional groups provides specific management recommendations for forest meadows that can be beneficial for cavity-nesting wasps hunting for aphids.
Bee nesting was not affected by mulching and the removal of all flowering vegetation in the meadows (hypotheses 1). The lack of response of bees may indicate that these high-altitude and forest adapted bees foraged either at times that were less affected by mulching (before June or in July or August), on fast regrowing flower species or outside the meadows, potentially in the surrounding forests, as was shown by previous studies in different landscape contexts (Rodríguez and Kouki 2015; Winfree et al. 2007). The species we found confirm these assumptions. Although we did not identify all reared imagos, we found specialists like Osmia parietina, foraging on fast regrowing Fabacea, Megachile lapponica, foraging on Epilobium sp., that may grow in patches not affected by the management commonly occurring along forest roads. Additionally, we found Osmia truncorum, a specialist on Asteracea which likely occurs at the highest abundance in forests (e.g. on Senecio ovatus). Bees in the genus Hylaeus can also do their foraging in trees/forests (Westrich 2018). Additionally, Eckerter et al. (2021) found, that cavity-nesting bee abundance in the same study area (northern black forest) was not affected by the amount of meadows area in the surroundings. Also, cavity-nesting bees depend on some nesting material (resin, leaves) which is obtained from forests (Rodríguez and Kouki 2015). Future studies, which examine the proportions of nesting resources and pollen collected from forest plants and meadow plants in bee nests could reveal the importance that both meadow and forest flowers have for the cavity-nesting bee communities in forest meadows.
The nest number of carnivore-hunting wasps (and more specifically spider-hunting wasps) was not affected by the mulching treatments (hypotheses 2). This might be explained by a lack of foraging activity of spider-hunting wasps in the meadows. It is more likely that spider-hunting wasps forage amongst forest vegetation, as especially forests providing complex vegetation structures, which is important for spiders (Brauckmann 2013; McDonald 2007). Although we did not identify all reared imagos the spider-hunting wasp Trypoxylon figulus is known to be strongly associated with forest habitats (Osorio et al. 2015) and was often found in our trap nests. Even though, spiders are also abundant in grasslands they might be non-target species to our observed wasp community, as Brauckmann (2013) found a negative effect on spider abundance in the herb layer by mulching compared to not mulching and Bornholdt et al. (1997) also found more spiders on not mulched meadows. A future study directly investigating the effect of mulching on predator species that rely on spiders as prey might shed some light on this question.
The number of herbivore-hunting wasp nests was reduced both by mulching once in September and 2 times during the growing period in the second sampling year (hypotheses 3). The wasp genus Passaloecus (hunting aphids) was most abundant and the genera Pemphredon (hunting aphids), Ancistrocerus (hunting caterpillars), Symmorphus (hunting beetle larvae), Nitela (hunting mainly barklice) and Rhopalum (hunting mainly barklice) contributed fewer individuals. Therefore, aphids were the main food resource of the observed community. As aphid-hunting wasps are highly mobile, we argue that the mulching did not kill the adult wasps directly but likely their larval food resources. The changing nest numbers suggest that meadow aphids were the preferred food resource for the observed aphid-hunting wasps which is supported by a study of Osorio et al. (2015). That means, the aphid-hunting wasps could probably not escape to the forest for larval food provisioning or produced fewer nests due to longer foraging flights. Therefore, mulching in September and twice a year, which can be expected to decrease aphid abundance, likely also disturbed aphid-hunting wasp nesting. As without disturbance through mulching, especially in August and September aphid abundances remain high (Chung et al. 1980). The spider abundance (Blick 2014, Bornholdt 1997) and the availability of pollen (Frankie et al. 2019, Salisbury 2015) declines towards the end of the growing period. Therefore, aphid-hunting wasps might still be nesting at the end of the growing season, while bees and other wasps have finished nesting earlier or possibly before the first mulching took place. We also expected the caterpillar- and beetle larvae-hunting wasp nest numbers to be negatively affected by mulching twice or in September, as insect larvae are negatively influenced by mulching (Humbert 2010). But this was not the case. As, insect larvae are also very abundant at the end of the growing season (Holmes et al. 1979) the larvae-hunting wasps on our examined forest meadows may also find their larval food in forests or due to their phenology earlier or even before the first mulching took place. Future studies on the responses of individual herbivore-hunting wasp species and their food resources to mulching over several years and taking seasonal activity patterns of species into account may confirm our observations and show the impact that mulching has across trophic levels.
Mulching in June, as we recommend for aphid-hunting wasps agrees with the current mulching recommendations which recommend mulching either in June/July or twice in June/July and August/September with the conservation of the overall vegetation community in mind (Schreiber et al. 2013). The reason behind this, is that late mulching leads to biomass accumulation and thereby inhibits the growth of light dependent plant species (Poschlod et al. 2013). With regards to mulching, the site conditions are important, e.g., for meadows which were historically kept open by grazing, machine mulching is no substitute for preserving the plant species community (Römermann et al. 2009). Additionally, late flowering plants are often adversely affected by early mulching (Römermann et al. 2009). Also, certain soils and climatic conditions are unfavourable for mulching (e.g., wet soils and cold air temperatures) (Poschlod et al. 2013). An alternative to mulching the whole grassland area in June, is mulching, or even mowing in strips. This might prove to be the best management strategy to conserve the overall plant and insect community (Hoste-Danyłow et al. 2010; Humbert et al. 2009).
If mulching is applied, the cuttings remain in the meadow (Schreiber et al. 2013). Therefore, it is often claimed that mulching leads to a biomass and nutrient accumulation (Schreiber et al. 2013). However, this was not the case in the 45 year long management experiment “Offenhaltungsversuche Baden-Württem-berg” (Brauckmann 2013; Schreiber et al. 2013). Our mulching experiment was too short in duration to answer this question for forest meadows. It took Doležal et al. (2011) five to six years to show the changes that occurred in the vegetation communities induced by mulching. Furthermore, the intra-annual variation in the number of bee nests, beetle-larvae hunting wasp nests and barklice-hunting wasp nests shows that there are potentially other factors such as climatic parameters that may influence populations even more than do food resources. To fully understand the effect of mulching of isolated forest meadows on bee and wasp populations long-term studies are required.
Our study focused on meadows completely surrounded by forests. Therefore, the management of the meadows alters the complete non-forest habitat. This scenario also likely occurs in patches of seminatural habitats, meadows, flower strips, field margins in agricultural landscapes surrounded by monotone habitats of low natural value. Therefore, our results might also apply to isolated habitats in agricultural landscapes but further research is needed for confirmation.
Conclusion
Mulching affects the studied functional groups of cavity-nesting insects on forest meadows differently. The high mobility of cavity-nesting insects may allow at least some groups, for example, bees to find resources outside the managed grassland that compensates for the temporary lack of resources due to mulching. However, aphid-hunting wasps were negatively affected. To which extent resource type and resource recovery after mulching contributes to cavity-nesting insect population dynamics and if strip-mulching could be an alternative strategy, needs to be investigated in further studies. For management implications, our data suggest that mulching of forest meadows in June produces the least severe effect for aphid-hunting wasps nest numbers. We therefore recommend mulching in June rather than in September or twice a year for protecting aphid-hunting wasps.
Data availability
No.
Code availability
No.
References
Aboling S (2003) Flora und äsung auf wildäckern der feldflur in niedersachsen. Zeitschrift Für Jagdwissen-Schaft 49(3):161–190
Bates D, Maechler M, Bolker B, Walker S, Bojesen RH, Singmann H, Dai B, Sheipl F, Grothendieck G, Green P, Fox J (2018) lme4: linear mixed-effects models using ‘Eigen’ and S4. R Found Stat Comput. https://doi.org/10.48550/arXiv.1406.5823
Blick, T. (2014) Analysis and remarks on the phenology of forest spiders in Hesse, Central Germany (Arachnida, Araneae), European Congress of Arachnology: 17
Bornholdt G, Brenner U, Hamm S, Kress JC, Lotz A, Malten A (1997) Zoologische untersuchun-gen zur grünlandpflege am beispiel von borstgrasrasen und goldhaferwiesen in der hohen rhön. Natur Landschaft 72(6):275–281
Brauckmann H-J (2013) Tierwelt der Versuchsflächen - Laufkäfer und Spinnen. In: Landesanstalt für Umwelt Baden-Württemberg (ed) Artenreiches Grünland in der Kulturlandschaft: 35 Jahre Offenhaltungsversuche Baden-Württemberg, 2nd edn. Regionalkultur, Heidelberg, pp. 314–332
Buse J, Eckerter T, Eichenseer P, Förschler MI, Oelmann Y, Georgi M (2018) Conservation value of small meadows in a forest-dominated landscape assessed for ground beetles (Coleoptera: Carabidae). Angewandte Carabidologie 12:49–56
Chung KH, Kwon SH, Lee YI (1980) Studies on the density of soybean aphids in different cultivars, planting dates and spacings. J Korean Soc Crop Sci 25:35–40
Cizek O, Zamecnik J, Tropek R, Kocarek P, Konvicka M (2012) Diversification of mowing regime increases arthropods diversity in species-poor cultural hay meadows. J Insect Conserv 16:215–226
Dicks LV, Breeze TD, Ngo HT, Senapathi D, An J, Aizen MA, Basu P, Buchori D, Galetto L, Garibaldi LA (2021) A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat Ecol Evol 5:1453–1461
Doležal J, Mašková Z, Lepš J, Steinbachová D, de Bello F, Klimešová J, Tackenberg O, Zemek F, Květ J (2011) Positive long-term effect of mulching on species and functional trait diversity in a nutrient-poor mountain meadow in Central Europe. Agr Ecosyst Environ 145(1):10–28
DWD (2019) Deutscher Wetterdienst: Climate Data Cenet (CDC): Rasterprodukts. https://cdc.dwd.de/portal/201810240858/mapview. Accessed 20 February 2019
Ebeling A, Hines J, Hertzog LR, Lange M, Meyer ST, Simons NK, Weisser WW (2018) Plant diversity effects on arthropods and arthropod-dependent ecosystem functions in a biodiversity experiment. Basic Appl Ecol 26:50–63
Eckerter T, Buse J, Bauhus J, Förschler MI, Klein AM (2021) Wild bees benefit from structural complexity enhancement in a forest restoration experiment. For Ecol Manage 496:119412
Erlacher G, Völk FH (2003) Änderungen der Waldstruktur im Staatswald-Neue Herausforderungen für die Bejagung des Schalenwildes. Bundesanstalt für Alpenländische Landwirtschaft, Gumpenstein (Hrsg.): Tagung für die Jägerschaft:27–37
Förschler M (2015) Nationalpark Schwarzwald–eine erste Gebietsgliederung. Naturschutzinfo:33–35
Frankie G, Feng I, Thorp R, Pawelek J, Chase MH, Jadallah CC, Rizzardi M (2019) Native and non-native plants attract diverse bees to urban gardens in California. J Pollinat Ecol. https://doi.org/10.26786/1920-7603(2019)505
Gilhaus K, Boch S, Fischer M, Hölzel N, Kleinebecker T, Prati D, Rupprecht D, Schmitt B, Klaus VH (2017) Grassland management in Germany: effects on plant diversity and vegetation composition. TUEXENIA 37:379–397
Hallmann CA, Ssymank A, Sorg M, de Kroon H, Jongejans E (2021) Insect biomass decline scaled to species diversity: general patterns derived from a hoverfly community. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2002554117
Harris AC (1994) Ancistrocerus gazella (Hymenoptera Vespoidea: Eumenidae): a potentially useful biological control agent for leafrollers Planotortrix octo, P. excessana, Ctenopseustis obliquana, C. herana, and Epiphyas postvittana (Lepidoptera: Tortricidae) in New Zealand. N Z J Crop Hortic Sci 22:235–238
Hartig, F. (2017) DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.1, 5
Holmes RT, Schultz JC, Nothnagle P (1979) Bird predation on forest insects: an exclosure experiment. Science 206:462–463
Hoste-Danyłow A, Romanowski J, Żmihorski M (2010) Effects of management on invertebrates and birds in extensively used grassland of Poland. Agr Ecosyst Environ 139(1–2):129–133
Humbert J-Y, Ghazoul J, Walter T (2009) Meadow harvesting techniques and their impacts on field fauna. Agr Ecosyst Environ 130(1–2):1–8
Humbert J-Y, Ghazoul J, Sauter GJ, Walter T (2010) Impact of different meadow mowing techniques on field invertebrates. J Appl Entomol 134:592–599
IPBES (2019) Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the intergovernmental science-policy platform on biodiversity and ecosystem services. In: Díaz S, Settele J, Brondízio ES, Ngo HT, Guèze M, Agard J, Arneth A, Balvanera P, Brauman KA, Butchart SHM, Chan KMA, Garibaldi LA, Ichii K, Liu J, Subramanian SM, Midgley GF, Miloslavich P, Molnár Z, Obura D, Pfaff A, Polasky S, Purvis A, Razzaque J, Reyers B, Roy Chowdhury R, Shin YJ, Visseren-Hamakers IJ, Willis KJ, Zayas CN (eds) IPBES secretariat. Ecology and Society, Bonn, p 56
Klein A-M, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B: Biol Sci 274:303–313
Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, Ritchie ME, Howe KM, Reich PB, Siemann E (1999) Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol Lett 2:286–293
Krombein KV (1967) Trap-nesting wasps and bees: life histories, nests and associates. Smithsonian Inst. Press, Washington, DC, p 570
LUBW (2006) Klimaatlas des Landes Baden-Württemberg. Karlsruhe
McDonald B (2007) Effects of vegetation structure on foliage dwelling spider assemblages in native and non-native Oklahoma grassland habitats:85–88
Mupepele A-C, Böhning-Gaese K, Lakner S, Plieninger T, Schoof N, Klein A-M (2019) Insect conservation in agricultural landscapes: an outlook for policy-relevant research. GAIA-Ecol Perspect Sci Soc 28:342–347
Osorio S, Arnan X, Bassols E, Vicens N, Bosch J (2015) Local and landscape effects in a host–parasitoid interaction network along a forest–cropland gradient. Ecol Appl 25:1869–1879
Petrak, M., Markett, P., Neitzke, A., Hochwildring, D., Cramer, F. U. (2015) Gestaltung von Wildwiesen und Äsungsflächen - Gemeinsame Aufgaben für Jagd und Naturschutz
Petrak M (2003) Zertifizierung von Wald, Wild und Jagd: Eine Detailfrage macht Klärungsbedarf deutlich. LÖBF-Mitteilungen:42–45
Poschlod, P., Schreiber, K.-F., Mitlacher, K., Römermann, C., & Bernhardt‐Römermann, M. (2013) Ent‐wicklung der Vegetation und ihre naturschutzfachliche Bewertung. In Landesanstalt für Umwelt Ba-den-Württemberg (LUBW) (Ed.), Artenreiches Grünland in der Kulturlandschaft: 35 Jahre Offenhal-tungsversuche Baden-Württemberg (2nd ed., pp. 243–299). Heidelberg: regionalkultur
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rannap R, Kaart T, Pehlak H, Kana S, Soomets E, Lanno K (2017) Coastal meadow management for threatened waders has a strong supporting impact on meadow plants and amphibians. J Nat Conserv 35:77–91
Rodríguez A, Kouki J (2015) Emulating natural disturbance in forest management enhances pollina-tion services for dominant Vaccinium shrubs in boreal pine-dominated forests. For Ecol Manage 350:1–12
Römermann C, Bernhardt-Römermann M, Kleyer M, Poschlod P (2009) Substitutes for grazing in semi-natural grasslands–do mowing or mulching represent valuable alternatives to maintain vegeta-tion structure? J Veg Sci 20(6):1086–1098
Rypstra AL, Schmidt JM, Reif BD, DeVito J, Persons MH (2007) Tradeoffs involved in site selection and foraging in a wolf spider: effects of substrate structure and predation risk. Oikos 116:853–863
Salisbury A, Armitage J, Bostock H, Perry J, Tatchell M, Thompson K (2015) Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): should we plant native or exotic species? J Appl Ecol 52(5):1156–1164
Sánchez-Bayo F, Wyckhuys K (2021) Further evidence for a global decline of the entomofauna. Austral Entomol 60:9–26
Schreiber K-F, Brauckmann H-J, Broll G, Fabricius C, Krebs S, Poschlod P (2013) Entscheidungshilfen für die Landschaftspflege - Schlussfolgerungen aus den Offenhaltungsversuchen Baden-Württemberg. In: Landesanstalt für Umwelt Baden-Württemberg (ed) Artenreiches Grünland in der Kulturlandschaft: 35 Jahre Offenhaltungsversuche Baden-Württemberg, 2nd edn. Regionalkultur, Heidelberg, pp. 347–376
Schuch S, Bock J, Krause B, Wesche K, Schaefer M (2012) Long-term population trends in three grassland insect groups: a comparative analysis of 1951 and 2009. J Appl Entomol 136:321–331
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarli D, Ammer C, Bauhus J, Fischer M, Habel JC (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674
Staab M, Pufal G, Tscharntke T, Klein A-M (2018) Trap nests for bees and wasps to analyse trophic interactions in changing environments—a systematic overview and user guide. Methods Ecol Evol 9:2226–2239
Steffan-Dewenter I (2002) Landscape context affects trap-nesting bees, wasps, and their natural enemies. Ecol Entomol 27:631–637
Sullivan ER, Powell I, Ashton PA (2018) Long-term hay meadow management maintains the target community despite local-scale species turnover. Folia Geobot 53:159–173
Tomić Z, Bijedić Z, Vilotić D, Gačić DP (2010) Phytocenological research into the meadow associ-ations on forest hunting grounds of Serbia. Arch Biol Sci 62(2):363–372
Tscharntke T, Gathmann A, Steffan-Dewenter I (1998) Bioindication using trap-nesting bees and wasps and their natural enemies: community structure and interactions. J Appl Ecol 35:708–719
Tschöpe T, Haußer T, Pojtinger, Berthold, Pojtinger, Wolfgang, Pojtinger, Hans, Finkbeiner W, Göckelmann C, Würth E, Schindler B, Klumpp W (2017) Umfrage zur Historie der Wildwiesen im Gebiet des Nationalpark Schwarzwaldes
Tschöpe T, Haußer T, Pojtinger, Berthold, Pojtinger, Wolfgang, Pojtinger, Hans, Finkbeiner W, Göckelmann C, Würth E, Schindler B, Klumpp W (2018) Umfrage zur Historie der Wildwiesen im Gebiet des Nationalpark Schwarzwaldes
Tylianakis JM, Tscharntke T, Klein AM (2006) Diversity, ecosystem function and stability of parasitoid-host interactions across a tropical gradient of habitat modification. Ecology 87:3047–3057
Völk, F. H. (1999) Äsungsflächen als Wildschadensprophylaxe? Möglichkeiten und Grenzen im Vergleich mit waldbaulichen Maßnahmen unter besonderer Berücksichtigung jagdkritischer Meinungen. Bun-desanstalt für alpenländische Landwirtschaft, 1–7
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: death by a thousand cuts. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2023989118
Westrich P (1996) Habitat requirements of central European bees and the problems of partial habitats. Linnean Society Symposium Series. Linnean Society:1–16
Westrich P (2018) Die Wildbienen Deutschlands. Stuttgart
Winfree R, Griswold T, Kremen C (2007) Effect of human disturbance on bee communities in a for-ested ecosystem. Conserv Biol 21(1):213–223
Acknowledgements
We thank the Black Forest National Park for help with the organization of the field work, providing historical- and geo-data. We also thank the LGFG (Landesgraduiertenförderung) and FAZIT- scholarship (scholarship from the Frankfurter Allgemeine Zeitung) for financial support and the hard-working field assistants Magdalena Pfau and Sarah Schöne and Jan Carl Matysiak for help in the lab. We thank Prof. Dr. Oliver Niehuis and Matthias Jäger for species identification and Bernhard Thiel for language corrections.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was funded by the LGFG and FAZIT scholarship.
Author information
Authors and Affiliations
Contributions
MG, AMK and SG developed the design of the study. MG established the trap nests, collected and organized the data sampling. MG, SN and AG analyzed the trap nests in the laboratory. MG analyzed the trap nest data with additional input by FF. MG wrote the manuscript with input and critical revision by all co-authors.
Corresponding author
Ethics declarations
Conflicts of interest
No.
Ethical approval
No.
Consent to participate
No.
Consent for publication
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Georgi, M.M., Fornoff, F., Gärtner, S.M. et al. Timing and mulching frequency affected the number of nests of cavity-nesting wasps that hunt for aphids in forest meadows. J Insect Conserv 26, 973–981 (2022). https://doi.org/10.1007/s10841-022-00442-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00442-y